eOrganic authors:
Tom Bilbo, Clemson University
J.C. Chong, Clemson Univerasity
Oscar Liburd, University of Florida
Rajagopalbabu Srinivasan, University of Georgia
Jim Walgenbach, North Carolina State University
David Lamie, Clemson University
Introduction
In 2018, a team of researchers from Clemson University, the University of Florida, the University of Georgia, and North Carolina State University were awarded a NIFA OREI grant to improve the biological control of spider mites, whiteflies and thrips in organic cucurbit and tomato systems in the southeastern U.S. This article summarizes the research objectives and findings of the project.
Objectives
The goal of this project was to improve augmentation and conservation of predatory natural enemies for the management of these key vegetable pests through the following two research objectives along with Extension and education programming to improve stakeholder knowledge and implementation of biological control.
1. Improve augmentative or inoculative releases of predatory mites to control spider mites, whiteflies, and thrips
- Determine optimal release timing of Phytoseiulus persimilis in tomato
- Determine optimal release timing of Amblyseius swirskii in squash
- Develop tomato-adapted strains of P. persimilis and A. swirskii to improve control of spider mites and western flower thrips on tomato
- Develop a rearing system for growers to rear both species of predators for release on small organic farms
- Examine compatibility of P. persimilis and A. swirskii with OMRI materials
- Assess the impact of predatory mite releases on vector dispersal and primary and secondary spread of the virus in tomato and squash
- Assess the economics of the biocontrol program
2. Identify and conserve endemic natural enemies of spider mites, thrips, and whiteflies
- Diversity survey of potential thrips, spider mite, and whitefly predators in tomato and squash cropping systems
- Examine non-target lethal and sublethal effects of OMRI pesticides on the three most common natural enemies, as determined by the survey
- Examine interactions between endemic predators and augmentatively released predatory mites
Summary of Findings
Developing and Rearing Tomato-adapted Predatory Mites (Drs. Jim Walgenbach and Tom Bilbo, North Carolina State University)
Field studies in North Carolina demonstrated that Phytoseiulus persimilis predatory mites can be successfully used in staked tomatoes to manage twospotted spider mites. Our experiments developed an inoculative approach where predators are released only in the section(s) of tomato fields where spider mites first establish early in the cropping period. This early, targeted release uses the 20,000 predators/acre inundative rate established in strawberries but in only 5-10% of the field, greatly reducing input requirements. Predators must be released early in a spider mite infestation, otherwise the prey to predator ratio is too large and spider mite populations grow too large for too long resulting in yield loss. Predators take several weeks to establish and outpace spider mites, with suppression and eradication typically occurring 3-5 weeks post-release. Predatory mites can only be effective when compatible insecticides/acaricides are used, and we determined that the selective acaricide bifenazate could be used in conjunction with P. persimilis for maximum spider mite suppression, while broad-spectrum insecticides such as pyrethroids are highly disruptive of predator performance.
We also investigated whether the host plant source influences the efficacy of P. persimilis. We conducted a large-scale on-farm experiment to compare spider mite management between a commercially obtained population reared on bean leaves and a population collected from tomato fields and reared on tomatoes. Tomato-adapted P. persimilis provided the best suppression overall, but bean-adapted predators still significantly reduced spider mites compared to the no predator control.
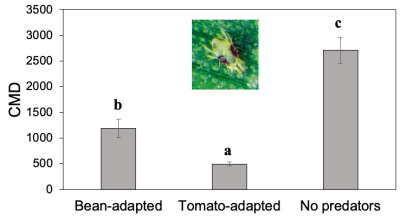
Fig. 1. Efficacy of bean- vs tomato-adapted P. persimilis for managing twospotted spider mites (measured as cumulative mite days, CMD) in a replicated experiment on a commercial staked tomato farm in North Carolina (manuscript in preparation).


Fig 2a (Left): Tomato foliage heavily damaged by twospotted spider mites in North Carolina. Fig 2b (Right): Numerous P. persimilis (red) on tomato leaf, after nearly eliminating all spider mites. Photo credit Tom Bilbo, Clemson University.
A grower-friendly rearing system was developed for tomato-adapted P. persimilis. This system requires three locations: one to grow uninfested plants, one for spider mite-infested plants, and one for predator-rearing plants prior to releasing in fields. When tomato transplants have approximately three leaves spider mites are evenly infested and allowed to increase for 3-5 weeks until webbing first appears. Predators are then introduced and require 3-4 weeks to reach maximum population size with spider mites still present to avoid premature predator dispersal. This approach was able to achieve up to 300 P. persimilis per tomato plant, although 100-200 was more common.

Fig. 3: Hoophouse used for rearing P. persimilis on trays of tomato plants. Photo credit: Tom Bilbo, Clemson University.
Presentations at numerous field days, grower meetings, webinars, and scientific conferences has revealed significant interest and enthusiasm in using predatory mites for managing spider mites, and for continuing this field of research to provide effective and economical biological control options for key vegetable pests.
For more information on this research, view the eOrganic webinar: How to manage spider mites in tomatoes with predatory mites
Effects of the Predatory Mite Amblyseius swirskii on Whitefly Bemisia tabaci Populations in Organic Squash (Dr. Oscar Liburd, University of Florida)
Biological control agents can be released alone or in modified landscapes within vegetable crops to enhance biological control services. Therefore, companion plants may improve the performance of a key mite predator, Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) in a cropping system where there are generalist herbivores such as the sweetpotato whitefly, Bemisia tabaci Genn. (Hemiptera: Aleyrodidae). Bemisia tabaci is a key insect pest of vegetable crops and ornamentals throughout the world. In Florida, it transmits important diseases in cucurbits (squash), including the cucurbit leaf crumble virus. In field experiments, we evaluated the effectiveness A. swirskii on managing B. tabaci populations. Furthermore, we measured the establishment and distribution A. swirskii in field grown squash in the presence of an insectary plant, sweet alyssum, Lobularia maritima.
Two treatments were investigated: One treatment had squash intercropped with sweet alyssum and the release of A. swirskii only on squash plants, a second treatment (control) had squash with sweet alyssum, but no A. swirskii were released onto the squash. We found a significant reduction in the number of whitefly eggs, immatures (nymphs) and adults in plots treated with A. swirskii compared to the control (Fig. 1). Amblyseius swirskii was able to spread throughout the squash field but did not become established in the sweet alyssum. Numerically, there was a reduction in the spread of leaf crumble virus in the treatment plot but this was not significantly different to the control.
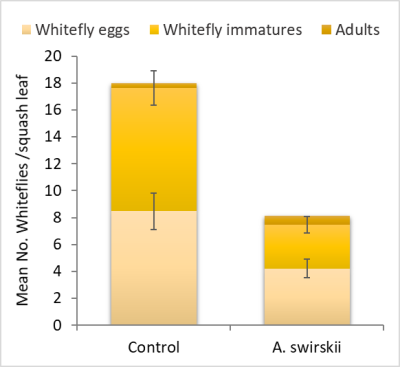
Fig. 4. Population of whiteflies (immatures, eggs and adults) in zucchini squash intercropped with sweet alyssum with and without a release of predatory Amblyseius swirskii.
Predators can affect whitefly populations in two ways, by reducing their density (consumptive effects) or by changing their behavior, physiology, or other phenotypic traits (non-consumptive effects). Predator-prey interactions regarding whiteflies provide a unique opportunity to study non-consumptive pathways because the eggs and nymphs are susceptible, but the adults are more difficult to manage with predators. Understanding how A. swirskii and a generalist predator, Delphastus catalinae (Coleoptera: Coccinellidae), interferes with whitefly ability to oviposit and consequently reduce the number of offspring is critical for predicting the strength of biological control services. We hypothesized that the presence of one or more predator species interferes with the whitefly’s ability to oviposit, which is reflected in the eventual offspring. To assess the effect of predator species on whitefly behavior and oviposition, we used an experimental arena consisting of a plastic container (11 cm diameter × 14.5 cm high) that contained a water source (50 ml centrifuge tube, 2 cm diameter × 8.5 cm high) to keep the seedlings turgid and covered with a lid with a hole filled by voile fabric. In each container, we had approximately six whitefly females (ca. 10 d old from adult emergence) and two oviposition leaves (squash) in the presence, or absence (control) of a predator. The experiment was carried out by pairing one arena including a predator individually, i) two D. catalinae, ii) two A. swirskii or in combination iii) two D. catalinae + two A. swirskii and, iv) one arena without the predator (control). We found that Whitefly females showed different oviposition capacities due to the presence or absence of one or more predator species. The number of eggs laid in the presence of D. catalinae or A. swirskii + D. catalinae was lower than the control, and there was no difference between A. swirskii and the control (Figure 2). The number of eggs in the control and A. swirskii treatments was significantly higher, more than 2.6 times compared with D. catalinae and A. swirskii plus D. catalinae (Table 1). The same trend was observed for the number of 1st and 2nd instar nymphs. However, for 3rd and 4th instar nymphs and adults only the combination of both predators was significantly different from the control and A. swirskii treatments (Table 1).
Table 1. The mean number (±SEM) of Bemisia tabaci offspring after 24 h of exposure to the predators, Amblyseius swirskii and Delphastus catalinae.
| Treatments | Eggs | 1st instar | 2nd instar | 3rd instar | 4th instar | Adult |
| Control | 21.40 ± 3.52 a
| 20.80 ± 3.47 a
| 17.80 ± 3.38 a
| 14.89 ± 2.60 a
| 14.11 ± 2.37 a
| 14.13 ± 2.68 a |
| Amblyseius | 18.40 ± 1.29 a
| 17.60 ± 1.28 a
| 17.50 ± 1.31 a
| 16.00 ± 1.12 a
| 16.44 ± 1.14 a | 16.25 ± 1.25 a
|
| Delphastus | 8.80 ± 1.84 0 b
| 8.50 ± 1.87 b
| 8.30 ±1.91 b
| 8.63 ±2.45 ab
| 9.43 ± 2.62 ab
| 8.86 ± 2.62 ab
|
| A +D | 6.10 ± 1.38 b
| 5.60 ± 1.38 b
| 6.00 ± 1.45 b
| 6.00 ± 1.45 b
| 6.00 ± 1.45 b
| 5.89 ± 1.43 b |
Means within columns followed by the same case letters are not significantly (Tukey’s HSD test P ≤ 0.05).
Key findings:
- Amblyseius swirskii is capable of reducing Bemisia tabaci in field-grown squash.
- Intercropping sweet alyssum with zucchini squash can enhance overall biological control, although it is possible that A swirskii does not forage aggressively in sweet alyssum.
- Amblyseius swirskii can spread throughout the crop (up to 125 m) from the release area.
- Predation risk from Delphastus catalinae can alter the oviposition and nymphal development of Bemisia tabaci and this effect may be enhanced by the presence of a second predator, Amblyseius swirskii.
For more information on this research,view the eOrganic webinar: Effects of the predatory mite, Amblyseius swirskii and its biproducts on whitefly Bemisia tabaci populations
Evaluating the Efficacy of Predatory Mites on Viruses Transmitted by Whiteflies and Thrips (Dr. Rajagopalbabu Srinivasan, University of Georgia)
At the University of Georgia Tifton campus, trials were conducted to evaluate the efficacy of predatory mites on viruses transmitted by whiteflies. This was evaluated both in the presence and absence of host plant resistance in tomato and yellow squash.
Whitefly-transmitted viruses are arguably the most limiting problems in the fall production of vegetables such as tomato and squash. Whitefly-transmitted tomato yellow leaf curl virus (TYLCV) in tomato, and whitefly-transmitted cucurbit leaf crumple virus (CuLCrV) in squash were targeted for the fall trials. The trials assessed how the release of predatory mites could affect the spread of vectors, and how the release would influence virus spread in the presence of a TYLCV-susceptible cultivar and a TYLCV-resistant cultivar. No CuLCrV resistance was available for squash, so one susceptible cultivar was evaluated. The release of predatory mites, A. swirski, in multiple forms, namely application at the base of the plant, on the foliage, and using sachets was undertaken in both trials. Additionally, a thrips-TSWV trial was conducted in the spring season using tomato under the same conditions with TSWV-susceptible and resistant cultivars.
Results indicated that the application or release of predatory mites were at times (some treatments) effective in suppressing whitefly and thrips populations. The nymphs were more affected by the releases of predatory mites than adults, especially in the case of whiteflies. However, this dent in vector populations did not result in the reduction of virus incidence of all three viruses. The virus severity was not affected either. Consequently, most susceptible plants did not produce any marketable yields. Host resistance in combination with predatory releases could present a more viable option; however, the availability of resistant cultivars for most viruses in these systems is limited.

Fig. 5. Predatory mite application in a tomato-TSWV trial. Photo credit: Rajagopalbabu Srinivasan, University of Georgia.
For more information on this research, view the eOrganic webinar: Predatory mite as a biocontrol agent for the management of insect-transmitted viruses in vegetable production.
Natural Enemy Diversity Survey, Evaluating the Effects of Organic Insecticides and Fungicides on Natural Enemies (Dr. JC Chong, Clemson University)
At Clemson University, researchers completed surveys to find out which natural enemies of thrips, whiteflies, and spider mites were present in organic production fields in Florida, Georgia, North Carolina, and South Carolina. Preliminary results suggest that the numbers of natural enemies in organic fields are surprisingly low, although this was possibly attributed to the sampling methods used and/or the low abundance of certain pest species. (The occurrence of some predator species is density-dependent regarding prey, so fewer prey equates to fewer natural enemies). The most common natural enemies were spiders, lady beetles, ground beetles, and predatory mites in the family Phytoseiidae. The abundance and family-level diversity of these natural enemies did not appear to correlate with the diversity of weeds or the crop system. Because of the low abundance, it was difficult to fully assess their impacts.
Due to the low abundance of the natural enemies in field surveys, we were not able to identify suitable natural enemy species as targets for lethal and sublethal effects analyses. Therefore, our approach to evaluating the impact of OMRI-listed insecticides and fungicides on natural enemies changed. Instead, we evaluated the impacts of these products on commercially available biological control agents, using commercially reared insects and mites as proxies for native or endemic natural enemies. Eight species of biological control agents of thrips, whiteflies, and spider mites were assessed, which include four predatory mite species (Amblyseius andersoni, Amblyseius swirskii, Neoseiulus californicus, and Phytoseiulus persimilis), lacewing larvae (Chrysoperla rufilabris), minute pirate bug (Orius insidiosus), and predatory beetles (Delphatus catalinae and Stethorus punctillum). Nine OMRI-listed insecticides and fungicides (plus water as the untreated control) were evaluated: azadirachtin (EcoGarden), Bacillus subtilis (Serenada ASO), Bacillus thuringiensis (DiPel DF), Chromabacterium subtsugae (Grandevo WDG), copper hydroxide (Kocide 3000), cuprous oxide (Nordox 75 WG), hydrogen peroxide + peracetic acid (OxiDate 2.0), pyrethrins (PyGanic Specialty), and spinosad (Entrust SC). All insecticides and fungicides were applied at the highest label rates and insects were exposed to the fresh residue.
Results demonstrate that, although some insecticides had significant negative impacts on natural enemies’ survival, other insecticides had mixed results. Mortalities of all natural enemy species were extremely high after contact with fresh residue of azadirachtin, pyrethrins and spinosad; therefore, growers who use these products should expect significant reduction in the abundance of natural enemies immediately after using these insecticides and highlight that even OMRI-registered insecticides carry risks for nontarget organisms. Eggs produced by minute pirate bugs were lower on plants treated with azadirachtin and pyrethrins. Copper fungicides are compatible with larger predators (minute pirate bugs, predatory beetles, and lacewing larvae) but no predatory mites. Bacillus subtilis, B. thuringiensis, C. subtsugae, and hydrogen peroxide + peracetic acid are compatible with all natural enemy species.
Growers may expect different outcomes for the natural enemy populations in their fields depending on the products used and the natural enemy groups of interest. They likely will not see a reduction in abundance and impact of natural enemies after the use of Bacillus subtilis, B. thuringiensis, C. subtsugae, and hydrogen peroxide + peracetic acid, but they may notice a significant drop in natural enemy abundance immediately after the applications of azadirachtin, spinosad, and pyrethrins. Previous research suggests that azadirachtin and pyrethrins generally have short residual toxicity; therefore, natural enemies may be able to repopulate the fields within a few days. Spinosad generally has longer residual toxicity so it may take a week or more before natural enemies fully repopulate the fields after application. Copper fungicides should not be used when the goal is to conserve predatory mites, but they are expected to have low impact on larger natural enemies.
For more information on this research, view the eOrganic webinar: Compatibility of Selected OMRI-listed Insecticides and Fungicides with Biological Control
Economic analysis (Dr. David Lamie, Clemson University)
Research farm-based experiments typically take place in rather unique circumstances not typically available in commercial farming operations. Extrapolating results from controlled research farm-based experiments to full scale commercial farming operations involves risk and uncertainty. Even so, we must start somewhere, and experimental trials at the research plot level allow the industry to assess the efficacy of new approaches in a relatively less risky environment than full-scale deployment in the fields of commercial farmers.
Economic analysis of IPM strategies essentially boils down to the following factors:
- Changes in yield, as compared to the next best alternative production strategy. More or less product available for sale at prevailing market prices ultimately determines revenue.
- Changes in input requirements (including germplasm, nutrition, tillage, equipment, water, labor, management/technical) for new IPM-enhanced production regimes as compared to the next best alternative production strategy. Changes in input requirements, at prevailing prices, determines costs.
- Changes in product quality (due to IPM-enhanced production regime) that might affect the prevailing prices that buyers are willing to pay for the crop produced.
Interviews of project Principal Investigators were carried out to better understand possible causes of changes in crop yield, input requirements, and product quality. During these interviews, several interesting issues surfaced, with potential economic implications.
It was recognized that a more robust population of beneficial insects could be developed if overwintering sites were made available, and if adjacent landowners did not spray pesticides at important times in their lifecycles. The success of IPM strategies relying on the deployment of beneficial insects could largely be determined by the location of any given farm in relation to an array of adjacent activities. New producers interested in farming methods that make good use of beneficial predatory insects, could consider purchasing land adjacent to producers deploying similar strategies or land adjacent to forested or fallow lands.
There are numerous environmental factors that might influence the economic implications of specific IPM strategies. Actual weather conditions and their impact on timing of production activities, the production practices of adjacent land managers, ambient populations of both predator and pest insects, the effective persistence of the predator species, and disease resistance of crop varieties are among the potential factors. Therefore, it would take several years of data acquired under conditions where these factors can be identified and accurately measured in order to suggest robust patterns of economic viability of any specific IPM strategy. Once clear patterns of yield and/or produce quality improvements emerge within the realm of the biological aspects of this research it is a fairly straightforward matter to estimate the economic implications.
With yield and cost of production data, we can estimate the economic implications by scaling results to a full acre of production, the typical scale used for Extension crop budgets. However, this would likely be misleading and lead to inappropriate producer adoption decisions. The only seemingly clear economic impact results from these various trials are those from the University of Georgia, where it was found that diseased non-resistant plants produced no marketable product. Further research could include repeated trials under similar conditions, taking care to measure yield and quality of production. Simultaneously, price data for important inputs should be collected over time to begin to measure trends in IPM-oriented production costs. Further, more work could be done to imagine projects, programs, and associated policies to foster cooperation amongst both organic and non-organic producers in order to help create more universal biodiversity at the regional level that might allow for the building of populations of beneficial predatory insects.
Conclusions
This project has developed new biological control tactics for thrips, mites, and whiteflies while also identifying possible limitations to these strategies. Spider mites can be successfully managed in tomatoes using the predatory mite Phytoseiulus persimilis but predator host plant source may influence outcomes. Amblyseius swirskii did not perform well in tomato production but could successfully suppress whiteflies and thrips in squash. The presence of certain predators, such as Delphastus catalinae, can provide added benefits such as non-consumptive effects influencing pest oviposition. More research in this area will better elucidate the influence of individual vs. predator combinations.
Predatory mite strategies explored in this study did not reduce virus incidence or severity, which highlights the importance of using integrated approaches for virus management including cultural controls and breeding for host plant resistance. Many biocontrol agents are available from commercial suppliers but this project demonstrated the feasibility for growers to rear their own predatory mites.
The success in deploying and conserving natural enemies is directly related to the use of compatible pesticides. This project showed that even some OMRI-listed pesticides can be toxic to certain natural enemies, so pesticides should be used judiciously especially when more sensitive natural enemy species and life stages are present. It will be important for future research to further investigate biological control options for thrips and whiteflies in tomatoes, where plant characteristics limit natural enemy survival and performance. The successful integration of selective insecticides and biological control—as well as outreach and education making the best strategies known to stakeholders—will be the key to achieving season long suppression of pests and profitable and sustainable vegetable systems in the southeastern US.
IMPORTANT: Before using any pest control product in your organic farming system:
- read the label to be sure that the product is labeled for the crop and pest you intend to control, and make sure it is legal to use in the state, county, or other location where it will be applied,
- read and understand the safety precautions and application restrictions, and
- make sure that the brand name product is listed in your Organic System Plan and approved by your USDA-approved certifier. If you are trying to deal with an unanticipated pest problem, get approval from your certifier before using a product that is not listed in your plan—doing otherwise may put your certification at risk.
Note that, although OMRI and WSDA lists are good places to identify potentially useful products, all products that you use must be approved by your certifier. For more information on how to determine whether a pest control product can be used on your farm, see the related article, Can I Use This Input On My Organic Farm?
Funding for this project was provided by NIFA OREI Grant 2018-51300-28425. Learn more about the project at https://eorganic.info/biocontrol



