eOrganic authors:
Matthew S. Jones, Washington State University
William E. Snyder, Wasnington State University
Introduction
Many organic vegetable farmers apply composted manure to enhance soil fertility, and some include livestock in their farming operations. All farms are visited by birds, deer, or other wildlife—all of which represent routes for animal feces to come in contact with fresh produce. This poses a risk to food safety in the rare cases that these feces are contaminated with human pathogens such as harmful strains of Salmonella, Listeria, Campylobacter, or Escherichia coli (Behling, 2010; Rasmussen and Casey, 2001). In turn, outbreaks of foodborne pathogens endanger human health and bring financial and legal risks to growers (Beretti and Stuart, 2008).
Fortunately, nature has provided a poop removal crew that can quickly remove fresh feces before it has a chance to contaminate produce. These creatures can also speed the release of plant-available nutrients contained in animal manure. Key among these are the dung beetles (families Scarabaeidae and Geotrupidae), who specialize in consuming fresh feces as larvae and adults. These insects play an important role in manure processing by consuming, burying, and breaking up the waste that both livestock and wildlife may deposit on farms (Doube, 1990; Menéndez et al., 2016). Here we focus on the benefits of dung beetles to vegetable and pasture production, the beetles’ feeding behaviors, and how to recognize a few key species likely to be seen on West Coast farms. Additionally, we make suggestions for how farmers can conserve dung beetles to maximize the many benefits that they offer.
Benefits of Dung Beetles
Dung beetles offer numerous benefits, including:
Suppression of human and livestock pathogens: By feeding on fresh feces and using it to provision their nests, dung beetles suppress dung-dwelling human and livestock parasites and pathogens (Nichols et al., 2008). Indeed, calves grazing on pasture with healthy dung beetle populations can have 75% fewer parasites (Fincher, 1975). Likewise, dung beetles can also kill pathogenic E. coli when they bury dung (Jones et al., 2015), making it less likely for these pathogens to contaminate produce. By eating both parasites and human pathogens, dung beetles can greatly improve human and livestock health. It should be noted that dung beetles feed on fresh feces, so pathogens found in improperly composted manure may be less likely to be consumed by dung beetles.
Improved soil hydrological properties: Many dung beetle species bury dung under the soil as food for their larvae (see Feeding Behavior below). This digging activity creates holes in the soil that increases permeability, aerating the soil (Bang et al., 2005) and allowing water to soak in instead of running off the surface (Brown et al., 2010).
Enhanced soil nutrient cycling: By burying freshly-deposited feces, dung beetles move nutrient-rich organic material to where plant roots can reach it and where it can feed other beneficial soil organisms (Bang et al., 2005; Manning et al., 2016). This also instigates micro-organismal and chemical changes in the upper soil layers, which accelerates ammonification, nitrification, denitrification, and nitrogen (N2) fixation (Yokoyama et al., 1991).
Reduced greenhouse gas emissions: By aerating and burying cattle dung pats on pasture, Slade et al. (2016) found that dung beetles could lower emission of the important greenhouse gas methane by up to 12%. Unfortunately, this same study found that conventional feedlots had few dung beetles, minimally reducing greenhouse gas by as little as 0.05%.
Reduced populations of pest flies: Some dung-breeding flies are pests of cattle, feeding on blood or around the cows’ eyes, mouth, and nostrils (Haufe, 1987). These pests retard cattle growth and are expensive to control (Byford et al., 1992). Fortunately, dung beetles can bury feces before fly eggs and larvae have a chance to develop (Bishop et al., 2005). This means that dung beetles are important natural controls of pest flies.
Feeding Behavior
Dung beetles have three different lifestyles: rollers (telecoprids), tunnelers (paracoprids), and dwellers (endocoprids) (Fig. 1). Rollers form the pat into balls that are rolled to a suitable site and buried. Tunnelers consume the dung pat and burrow into the soil beneath the pat. Manure dwellers consume the manure pat and deposit their eggs either in the same place or in the soil adjacent to the pat.
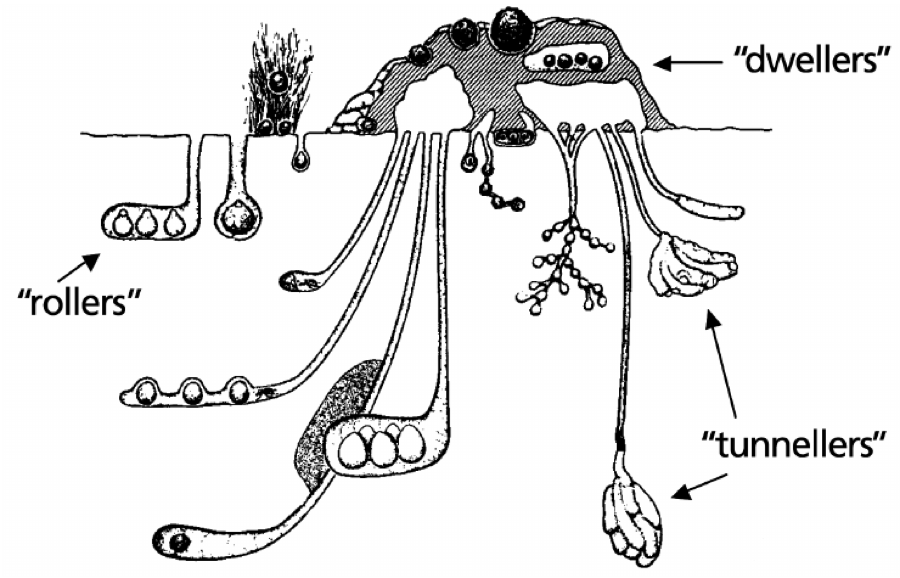
Figure 1. Cross section of a dung pat showing the three dung beetle nesting types: dwellers, tunnelers, or rollers. Photo Credit: Kevin Floate, Agriculture and Agri-food Canada, 2011.

Figure 2. The tumblebug (Canthon imitator) is a good example of a roller (telecoprid) species. Photo Credit: Whitney Cranshaw, Colorado State University, Bugwood.org.
Figure 3. Holes like this in cattle manure indicate dung beetle feeding. Photo Credit: Debra Murphy, realagriculture.com.
Management
Many of the same chemicals used to kill internal parasites of conventional livestock also harm or kill dung beetles (O'Hea et al., 2010; Beynon et al., 2012; Floate et al., 2005; Verdú et al., 2015). This means that producers trying to kill harmful livestock parasites with chemicals may also be accidentally killing beneficial dung beetles (Wall and Strong, 1987). While organic farmers are allowed to vaccinate livestock, the routine use of antibiotics, harmful to dung beetles, is prohibited. Eliminating these chemicals by farming organically is a great way to conserve dung beetles and maximize the benefits these beetles offer.
For organic farmers wanting to maximize the benefits of dung beetles, a few guidelines are:
- Take care not to overgraze pastures, which may reduce vegetation cover and increase soil exposure, and has been shown to result in high soil loss and compaction (Negro et al., 2011). This may directly disturb dung beetles, modify their habitat, or influence microhabitat requirements for larval development of the different functional groups—especially telecoprids and paracoprids—which require specific soil characteristics for burying dung and building nests (Bertone et al., 2006; Negro et al., 2011).
- If possible, maintain a diversity of ungulate species, which will increase dung beetle diversity and improve dung decomposition (Hutton and Giller, 2003).
For conventional farmers contemplating a transition to organic production and conventional farmers interested in reducing their impact on dung beetles, a few guidelines are:
- Rotate stock around fields and allow fields to fallow three weeks between grazing of the same animal species to help break parasite cycles in fields without using chemicals.
- Treat only livestock known to have parasites, rather than using preventative antibiotic treatments.
- Use chemicals less toxic to dung beetles, especially when cattle are out on pasture. See Beynon (2016) for more guidance on how to do this.
- Do not under-dose animals. Parasiticides are not effective unless the right dose is applied.
- Do not move treated animals onto fresh pasture immediately after treatment, and always ensure there is some untreated dung for beetles to eat.
Identification of Common Species
Figure 4. Canthon simplex (LeConte, 1857), commonly known as a “Tumblebug,” is a roller (telecoprid) species that is 5-9mm long. This species is plain black and very smooth. Also look for the serrated edge on the front of its head (as seen in the photo). This species can be found during the spring and summer throughout most of the U.S. West Coast. Photo Credit: Gary Griswold, bugguide.net.

Figure 5. Onthophagus taurus (Schreber 1759), commonly known as the “Bull Headed Dung Beetle,” is a tunneler (paracoprid) species that is 6.0-11.5mm long. This species is all black, sometimes with a greenish sheen. Males often have a pair of long horns sweeping back off their head (like a bull). This species can be found from spring through autumn throughout the United States. More information about this species can be found at: http://bugguide.net/node/view/23972. Photo Credit: Frank Guarnieri, bugguide.net.
Figure 6. Onthophagus nuchicornis (Linnaeus 1758) is a tunneler (paracoprid) species that is 6-8mm long. This species has a gold back speckled with black dots. Males often have a single spine-like horn and females have a ridge across the base of their head. This species can be found from early spring until late autumn from southern Oregon (United States) to Canada, as well as in northern latitudes across the United States. More information can be found at: http://bugguide.net/node/view/29475. Photo Credit: Emmy Engasser, Hawaiian Scarab ID, USDA APHIS ITP, Bugwood.org.
Figure 7. Aphodius fimetarius (Linnaeus, 1758) is a dweller (endocoprid) species that is 5-8mm in length. This species has ribbed, bright orange-red outer wings with a black head and thorax. This species is most likely to be found in the spring and autumn throughout the United States. Photo Credit: Emmy Engasser, Hawaiian Scarab ID, USDA APHIS ITP, Bugwood.org.
Additional Resources
Team Scarab at the University of Nebraska State Museum is the absolute taxonomic guru for help identifying obscure and interesting dung beetles. They maintain a large portion of the Smithsonian Museum’s dung beetle collection and can be found at: https://unsm-ento.unl.edu/scarabcentral.html.
Information regarding other (non-dung-feeding) beneficial beetles can be found at: http://ento.psu.edu/extension/factsheets/ground-beetles.
References and Citations
- Arnett, R. H., M. C. Thomas, P. E. Skelley, and J. H. Frank (eds.). 2002. American beetles. Volume 2: Polyphaga: Scarabaeoidea through Curculionoidea. CRC Press, Boca Raton, FL.
- Bang H. S., J-H. Lee, O. S. Kwon, Y. E. Na, Y. S. Janga, and W. H. Kim. 2005. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Applied Soil Ecology 29: 165—171. (Available online at: https://doi.org/10.1016/j.apsoil.2004.11.001 (verified 13 Apr 2023).
- Behling, R. G., J. Eifert, M. C. Erickson, J. B. Gurtler, J. L. Kornacki, E. Line, R. Radcliff, E. T. Ryser, B. Stawick, and Z. Yan. 2010. Selected pathogens of concern to industrial food processors: Infectious, toxigenic, toxico-infectious, selected emerging pathogenic bacteria. p. 5—61. In J. L. Kornacki (ed.) Principles of microbiological troubleshooting in the industrial food processing environment in food microbiology and food safety. Springer-Verlag New York.
- Beretti, M., and D. Stuart. 2008. Food safety and environmental quality impose conflicting demands on Central Coast growers. California Agriculture 62: 68–73. (Available online at: https://vric.ucdavis.edu/pdf/food_safety&environmental_quality_impose_conflicting.pdf (verified 13 Apr 2023).
- Bertone, M., W. Watson, M. Stringham, J. Green, S. Washburn, M. Poore, and M. Hucks. 2006. Dung beetles of central and eastern North Carolina cattle pastures. (Available online at: https://cefs.ncsu.edu/wp-content/uploads/bertoneguidetoncdungbeetles.pdf?x47549 (verified 13 Apr 2023).
- Beynon, S. A., D. J. Mann, E. M. Slade, and O. Lewis. 2012. Species-rich dung beetle communities buffer ecosystem services in perturbed agroecosystems. Journal of Applied Ecology 49: 1365–1372. (Available online at: https://doi.org/10.1111/j.1365-2664.2012.02210.x (verified 13 Apr 2023).
- Beynon, S. A. 2016. Fact sheet 2: Sustainable use of wormers and other parasiticides for cattle, sheep, and horses [Online]. Dung Beetles Direct. Available at: https://www.thebugfarm.co.uk/research-farm/dung-beetles-direct/ (verified 13 Apr 2023).
- Bishop, A. L., H. J. McKenzie, L. J. Spohr, and I. M. Barchia. 2005. Interactions between dung beetles (Coleoptera: Scarabaeidae)and the arbovirus vector Culicoides brevitarsis Kieffer (Diptera:Ceratopogonidae). Australian Journal of Entomology 44: 89–96. Available online at: https://doi.org/10.1111/j.1440-6055.2005.00455.x (verified 13 Apr 2023).
- Brown, J., C. H. Scholtz , J.-L. Janeau, S. Grellier, and P. Podwojewski. 2010. Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties. Applied Soil Ecology 46: 9–16. (Available online at: https://doi.org/10.1016/j.apsoil.2010.05.010 (verified 13 Apr 2023).
- Byford, R. L., M. E. Craig, and B. L. Crosby. 1992. A review of ectoparasites and their effect on cattle production. Journal of Animal Science 70: 597–602. (Available onlne at: https://doi.org/10.2527/1992.702597x (verified 13 Apr 2023).
- Doube, B. M. 1990. A functional classification for analysis of the structure of dung beetle assemblages. Ecological Entomology 15: 371–383. (Available online at: https://doi.org/10.1111/j.1365-2311.1990.tb00820.x (verified 13 Apr 2023).
- Eaton, E. R., and K. Kaufman. 2007. Kaufman Field Guide to Insects of North America. Houghton Mifflin Company, Boston, MA.
- Fincher, G. T. 1975. Effects of dung beetle activity on number of nematode parasites acquired by grazing cattle. Journal of Parasitology 61: 759–762. (Available online at: https://doi.org/10.2307/3279480 (verified 13 Apr 2023).
- Floate, K. D. 2011. Arthropods in cattle dung on Canada's grasslands. p. 71—88. In K. D. Floate (ed.) Arthropods of Canadian grasslands (Volume 2): Inhabitants of a changing landscape. Biological Survey of Canada. (Available online at: https://volumesdirect.com/products/arthropods-of-canadian-grasslands-volume-2?pr_prod_strat=copurchase&pr_rec_id=d9129a32b&pr_rec_pid=5776476438694&pr_ref_pid=5782344925350&pr_seq=uniform (verified 13 Apr 2023).
- Floate, K. D., K. G. Wardhaugh, A.B.A. Boxall, and T. N. Sherratt. 2005. Fecal residues of veterinary parasiticides: Nontarget effects in the pasture environment. Annual Review of Entomology 50: 153–79. (Available online at: https://doi.org/10.1146/annurev.ento.50.071803.130341 (verified 13 Apr 2023).
- Haufe, W. O. 1987. Host–parasite interaction of blood feeding dipterans in health and productivity of mammals. International Journal of Parasitology 17: 607–614. (Available online at: https://doi.org/10.1016/0020-7519(87)90137-8 (verified 13 Apr 2023).
- Hutton, S. A., and P. S. Giller. 2003. The effects of the intensification of agriculture on northern temperate dung beetle communities. Journal of Applied Ecology 40:994–1007. (Available online at: https://doi.org/10.1111/j.1365-2664.2003.00863.x (verified 13 Apr 2023)
- Jones, M. S., S. Tadepalli, D. F. Bridges, V.C.H. Wu, and F. A. Drummond. 2015. Suppression of Escherichia coli O157:H7 by dung beetles (Coleoptera: Scarabaeidae) using the lowbush blueberry agroecosystem as a model system. PLoS ONE 10: e0120904. (Available online at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0120904 (verified 13 Apr 2023).
- Manning, P., E. M. Slade, S. A. Beynon, and O. T. Lewis. 2016. Functionally rich dung beetle assemblages are required to provide multiple ecosystem services. Agriculture, Ecosystems & Environment: 218: 87–94. (Available online at: https://doi.org/10.1016/j.agee.2015.11.007 (verified 13 Apr 2023).
- Menéndez, R., P. Webb, and K. H. Orwin. 2016. Complementarity of dung beetle species with different functional behaviours influence dung–soil carbon cycling. Soil Biology and Biochemistry 92: 142–148. (Available online at: https://doi.org/10.1016/j.soilbio.2015.10.004 (verified 13 Apr 2023).
- Negro, M., A. Rolando, and C. Palestrini. 2011. The impact of overgrazing on dung beetle diversity in the Italian Maritime Alps. Environmental Entomology 40: 1081–1092. (Available online at: https://doi.org/10.1603/EN11105 (verified 13 Apr 2023).
- Nichols, E., S. Spector, J. Louzada, T. Larsen, S. Amezquita, and M. E. Favila. 2008. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation 141: 1461–1474. (Available online at: https://doi.org/10.1016/j.biocon.2008.04.011 (verified 13 Apr 2023).
- O'Hea, N. M., L. Kirwan, P. S. Giller, and J. A. Finn. 2010. Lethal and sublethal effects of ivermectin on north temperate dung beetles, Aphodius ater and Aphodius rufipes (Coleoptera: Scarabaeidae). Insect Conservation and Diversity 3: 24–33. (Available online at: https://doi.org/10.1111/j.1752-4598.2009.00068.x (verified 13 Apr 2023).
- Rasmussen, M. A. and T. A. Casey. 2001. Environmental and food safety aspects of Escherichia coli O157:H7 infections in cattle. Critical Reviews in Microbiology 27:57–73. (Available online at: http://dx.doi.org/10.1080/20014091096701 (verified 13 Apr 2023).
- Slade, E. M., T. Riutta, T. Roslin, and H. L. Tuomisto. 2016. The role of dung beetles in reducing greenhouse gas emissions from cattle farming. Scientific Reports 6: 18140. (Available online at: 10.1038/srep18140 (verified 13 Apr 2023).
- Verdú, J. R., V. Cortez, A. J. Ortiz, E. González-Rodríguez, J. Martinez-Pinna, J.-P. Lumaret. 2015. Low doses of ivermectin cause sensory and locomotor disorders in dung beetles. Scientific Reports 5: 13912. (Available online at: https://doi.org/10.1038/srep13912 (verified 13 Apr 2023).
- Wall, R. and L. Strong. 1987. Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature 327: 418–421. (Available online at: https://doi.org/10.1038/327418a0 (verified 13 Apr 2023).
- Yokoyama, K., K. Hideaki, and T. Hirofumi. 1991. Paracoprid dung beetles and gaseous loss of nitrogen from cow dung. Soil Biology and Biochemistry 23: 643–647. (Available online at: https://doi.org/10.1016/0038-0717(91)90077-W (verified 13 Apr 2023).







