eOrganic author:
Dr. Mark Schonbeck, Virginia Association for Biological Farming
Introduction
Yellow nutsedge (Cyperus esculentus) is a major weed of vegetable and row crops in temperate and tropical regions around the world. Native to the Americas, yellow nutsedge is especially aggressive in irrigated crops that are maintained at high soil moisture levels (Ransom et al., 2009), and is considered a major weed of vegetables, corn, cotton, and peanuts in the southern United States (Holm et al., 1991; Webster, 2006).
Yellow nutsedge is a grass-like weed in the sedge family (Cyperaceae) with top growth 8–30 inches tall (Fig. 1a), and an extensive underground network of basal bulbs, roots, thin fibrous rhizomes, and tubers 0.4–0.8 inch long borne singly at the tips of rhizomes (Uva et al., 1997; Holm et al., 1991). The leaves are mostly basal, bright green to yellow-green, 0.1–0.35 inch wide with a prominent midrib, and tapering gradually toward the tips. Leaves may be as long as, or longer than, the culm (stem), which is triangular in cross section, and bears the inflorescence (flower head). The inflorescence is yellow-brown, golden, or straw colored, and consists of an umbel of spikes borne on stalks of unequal length (1–3 inches), subtended by leaflike bracts as long or longer than the spikes (Bryson and DeFelice, 2009) (Fig. 1b).

Figure 1. (a) Yellow nutsedge in bloom. (b) Closeup of yellow nutsedge inflorescence, showing umbel of spikes of variable length, and leaflike bracts longer than the spikes. Photo credits: (a) Howard F. Schwartz, Colorado State University, Bugwood.org; (b) Mark Schonbeck, Virginia Association for Biological Farming.
Biology
Tuber dormancy in yellow nutsedge is broken by chilling at 35–50 °F for several weeks. Sprouting begins as soil temperatures rise above 55 °F (Holm et al., 1991; Stoller and Wax, 1973), and is promoted by tillage. Washing tubers has enhanced germination by 8-fold (Tumbleson and Kommendahl, 1961), which suggests that a water soluble inhibitor contributes to tuber dormancy, and that percolation of moisture through the soil may play a role in promoting germination (Mohler and DiTommaso, unpublished). Although the majority of tubers either sprout or die during the first year after they are formed, some tubers located deeper in the soil profile can remain dormant and survive 2–4 years (Stoller and Wax, 1973; California Department of Food and Agriculture) .
Yellow nutsedge begins active growth in mid- to late spring (FIg. 2a). A determinate and fairly short rhizome emerges from the tuber and grows toward the soil surface. When the tip of the rhizome receives a light stimulus, a basal bulb forms about 0.4–0.8 inch behind the tip (Stoller et al., 1972). The shoot, consisting of a cluster of basal leaves, arises from this bulb. In the field, basal bulbs generally form within 0.4–1.6 inches of the soil surface (Fig. 2b), regardless of the depth of the tubers from which they arise (Mulligan and Junkins,1976; Stoller et al., 1972; Uva et al., 1997). A fibrous root system develops from basal bulbs and rhizomes.
As the initial plant develops, it remains attached to the mother tuber for up to 12 weeks (Stoller et al., 1972). Within four weeks after initial shoot emergence, new indeterminate rhizomes emerge from the basal bulb, grow 1–20 inches laterally, and form new basal bulbs and daughter plants (Fig. 2b, Fig. 3). The cycle repeats several times, with new shoots continuing to develop through July in the central U.S. (Jordan-Molero and Stoller, 1978); thus, the weed spreads exponentially in the absence of competition or control measures.

Figure 2. Yellow nutsedge in a home garden in central Virginia, photographed in late May. (a) Growing with snap bean (b) Plants exhumed to show basal bulbs about 1–1.5 inches below the soil surface, with fibrous roots growing down into the soil, and white rhizomes extending laterally to establish daughter plants. Photo credits: Mark Schonbeck, Virginia Association for Biological Farming.

Figure 3. Yellow nutsedge specimens collected in late May in Virginia, about a month after emergence: (a) still attached to the mother tuber; (b) sending out several rhizomes, two of which have already initiated daughter plants. Photo credits: Mark Schonbeck, Virginia Association for Biological Farming.
In temperate zone populations of yellow nutsedge, shortening daylength in late summer triggers flowering and tuber production. When daylength decreases to about 14 hours, rhizome tips begin to form tubers rather than new daughter plants. This has been reported to occur in late July in Ohio (Cardina et al., 2012), near the beginning of August in Illinois (Jordan-Molero and Stoller, 1978), and mid to late August in Canada (Mulligan and Junkins, 1976). While top growth slows, prolific tuber production continues until killing frost. Tubers are formed mostly throughout the top 6–10 inches of the soil profile, although a few may form as deep as 18 inches (Tumbleson and Kommedahl, 1961). The mature, starchy tubers contain about 40-60% dry matter.
Yellow nutsedge thrives in moist to wet conditions (Fig. 4), and is highly tolerant to flooding. It can be incredibly prolific in temperate climates and high-moisture soils. A single tuber has been observed to give rise to 1,900 shoots and 6,900 tubers within one year in Minnesota (Tumbleson and Kommedahl, 1961), and 1,700–3,000 shoots and 19–20 thousand tubers in irrigated fields in Oregon (Ransom et al., 2009), forming a dense patch about 6 feet across. Tuber dry weight reached an equivalent of about 4 tons per acre. In the Oregon study, yellow nutsedge spread several fold more slowly when the soil was allowed to dry out between infrequent irrigations of 1.0–1.4 inches as recommended for sugarbeet and wheat, than when the soil was maintained near field capacity through frequent small (0.3 inch) irrigations as recommended for onion production. Yellow nutsedge spreads more slowly in very hot, sunny climates such as the low desert of southeastern California, where a single tuber gave rise to about 50 tubers after one growing season (Wang et al., 2008).

Figure 4. A dense stand of yellow nutsedge infests the lowest and wettest part of this field (foreground), with much lighter populations in the better-drained parts of the field (background). Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Yellow nutsedge tubers are killed by exposure for 1–2 days to temperatures of 113–122 °F (Webster, 2003) or below 20 °F, although cold hardening may enhance freeze tolerance by a few degrees (Stoller and Wax, 1973). In Illinois, most tubers within 2 inches of the soil surface are winterkilled but the weed readily emerges from tubers located 4–8 inches deep, where temperatures extremes are buffered. Yellow nutsedge has successfully spread into southeastern Canada, where snow cover may further protect tubers from winterkill. Desiccation or exposure to direct sunlight accentuates thermal stresses, and tubers brought to the surface by tillage rapidly lose viability during dry weather (Tumbleson and Kommedahl, 1961). Intact yellow nutsedge is quite tolerant to drought because of its extensive fibrous root system (Holm et al., 1991).
Attempts to eradicate yellow nutsedge by soil solarization in Georgia have been thwarted by survival of tubers deeper in the soil profile (Webster, 2003). However, solarization has successfully controlled this weed in the low desert of California (Wang et al., 2008).
Yellow nutsedge tolerates moderate (20–30%) shade with little decrease in growth or tuber production, whereas 60–80% shade reduces total biomass by more than half, and tuber biomass by 75% or more (Jordan-Molero and Stoller, 1978; Keeley and Thullen, 1978; Santos et al., 1997). Although the weed compensates for shade by growing taller, and can form some tubers even under 94% shade, crop competition for light is recognized as an important tactic that can enhance the efficacy of cultivation (Keeley and Thullen, 1978).
Yellow nutsedge can form viable seeds by cross-pollination between different clones (populations of genetically identical plants arising from vegetative reproduction) (Fig. 5). As many as 1,500 viable seeds per plant have been reported, yet yellow nutsedge seedlings are rarely observed in the field (Holm et al., 1991; Mohler and DiTommaso, unpublished). The seedlings are extremely sensitive to desiccation, and can establish successfully only when the soil surface remains continuously moist (Lapham and Drennan, 1980). Although sexual reproduction is not a significant means of propagation, its occasional occurrence allows for genetic recombination and adaptation. Morphological and ecophysiological differences among yellow nutsedge populations have been documented (Mulligan and Junkins, 1976), and an unusually vigorous biotype has been reported in California (Tayyar et al., 2003).

Figure 5. Yellow nutsedge seeds. Photo credit: Ken Chamberlain, The Ohio State University, Bugwood.org.
Impacts on Crop Production
Yellow nutsedge competes severely against many crops for moisture and nutrients (Fig. 6), and against low-growing crops for light. Its sharp pointed rhizomes can penetrate the edible portion of root crops, rendering them unmarketable; severe crop losses can occur in potato (Holm et al., 1991). Crop competition from tall crops like corn and tomato, and heavy canopy formers like potato can suppress nutsedge that emerges several weeks after crop planting, whereas slow-starting crops like onion and cotton, and warm season crops planted early when the soil is still cool, are especially prone to nutsedge competition. Potato growers in Ontario have noted that the variety ‘Kennebec,’ which is characterized by vigorous top growth, reduces yellow nutsedge populations in the following year, compared to less vigorous varieties such as ‘Katahdan’ and ‘Irish Cobbler’ (Mulligan and Jenkins, 1976).
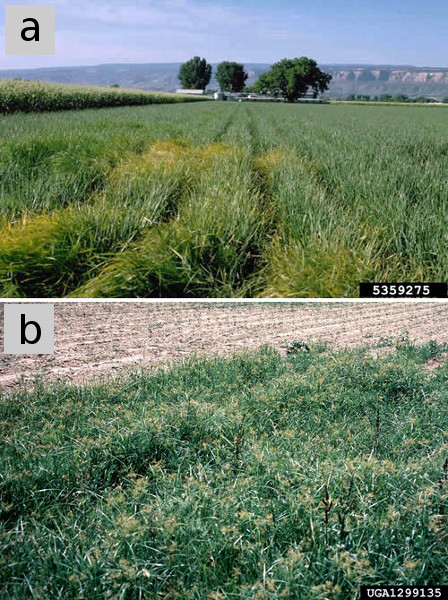
Figure 6. (a) A dense patch of yellow nutsedge in onion. (b) Heavy infestation of yellow nutsedge in cotton has virtually hidden the crop from view. Figure credits: (a) Howard F. Schwartz, Colorado State University, Bugwood.org; (b) John D. Byrd, Mississippi State University, Bugwood.org.
Allelopathic substances from yellow nutsedge have reduced growth of corn, soybean, and sweet potato in greenhouse trials (Drost and Doll, 1980; Peterson and Harrison, 1995). Although allelopathy has been cited as a factor in corn and soybean yield losses to yellow nutsedge (Cardina et al., 2012), more research is needed to verify this in field conditions.
A few crops may suppress yellow nutsedge through allelopathy. In field studies in South Carolina, sweet potato reduced yellow nutsedge biomass production by 90% through competition and allelopathy, without itself being hindered by the weed (Harrison and Peterson, 1991). Rye straw mulch and root residues in the soil left by roll-killing a winter rye cover crop can reduce yellow nutsedge growth (Mohler and DiTommaso, unpublished). In greenhouse trials, wild radish residues mixed into the potting soil reduced yellow nutsedge competitiveness against tomato and pepper (Norsworthy and Meehan, 2005a). However, yellow nutsedge tubers showed little sensitivity to several isothiocyanate allelochemicals released by decaying Brassica residues (Norsworthy and Meehan, 2005b).
Emerging shoots of yellow nutsedge readily penetrate organic mulches and the lighter grades of spun-fiber mulches. Other synthetic mulches, such as black polyethylene mulch can slow emergence and spread of yellow nutsedge by about half (Webster 2005a and 2005b); however, some plants pierce the mulch, which can complicate end-of-season mulch removal. Because shoot tips open in response to light, yellow nutsedge emerging under clear polyethylene may become trapped and heat-killed under the film, which weakens the mother tuber (Chase et al., 1998).
Yellow nutsedge did not show a strong response to fertilizer nitrogen (N) rate in Oregon (Ransom et al., 2009), and does not appear to compete more aggressively against crops at high levels of available nutrients in the Northeast (Mohler and DiTommaso, unpublished). However, in a Florida field trial, yellow nutsedge competed more aggressively against tomato at higher N application rates (Morales-Payan et al., 1997).
Yellow nutsedge thrives in moderately warm conditions with ample moisture. It is most troublesome in irrigated crops such as onion, which are maintained at high soil water potential (Ransom et al., 2009). One organic farmer in Arkansas has observed that yellow nutsedge is most abundant in fields with compacted soil and poor drainage, and becomes less so after organic management has improved soil drainage and soil quality (Josh Hardin, personal communication, 2009). Compared to its close relative purple nutsedge, yellow nutsedge was much easier to control and had far less impact on yields of irrigated broccoli in field trials in the low desert of southeastern California (Wang et al., 2008, Wang et al., 2009).
Management Strategies and Tactics for Organic Production
Timely tillage and cultivation during the initial flush of yellow nutsedge emergence in late spring can significantly weaken the weed. In experiments on Illinois populations, separating young plants (about 1 inch of shoot emerged above ground) from the mother tuber substantially reduced subsequent growth and spread (Stoller et al., 1972). Tubers that were germinated for 6 days at 77 °F in darkness expended over 60% of their dry weight, protein, carbohydrate, and oils to develop the sprout. Many tubers sprouted again after the initial sprout was removed, but the second sprout was much weaker (Stoller et al., 1972). Thus, the weed is most vulnerable to tillage in late spring, after tubers have sprouted but before the emerging shoot has begun to build up new reserves or initiate daughter plants. Tillage at this time and again in late summer (to disrupt formation of new tubers), combined with crop competition, have been found to control yellow nutsedge fairly well in the Northeast (Mohler and DiTommaso, unpublished).
One tillage operation will not control yellow nutsedge, and regrowth needs to be removed before the weed begins to rebuild underground reserves. Repeat cultivation before re-emerging shoots reach the 6-leaf stage (Russ and Burgess, 2009).
Yellow nutsedge that emerges in crops can be managed with cultivation. Farmers have had excellent results using a cultivator equipped with beet knives set about 1.5–2 inches below the surface to undercut the new growth and separate it from the tubers.
Repeated tillage alone may not be sufficient to eradicate a severe yellow nutsedge infestation. Two years of mechanical fallow throughout the frost-free period have reduced populations by 80–90% (Mulligan and Junkins, 1976; Tumbleson and Kommendahl, 1961); however the costs to soil quality and the production foregone through extended fallow make this approach impractical for most farms. Sustainable management of this weed requires an integrated strategy that subjects the weed to multiple stresses, especially during the critical times of initial emergence (mid–late spring) and tuber set (late summer). Key components include:
- Till or cultivate at critical times, working 1.5–2 inches deep to undercut basal bulbs when practical.
- Choose competitive crops and varieties, and manage crops to produce at least 80% shade between rows at ground level after the last cultivation.
- Plan crop rotations and schedule plantings and field operations to allow either cultivation or effective crop competition during the period of active nutsedge growth, usually mid–late spring through late summer.
- Follow spring vegetables with fast-growing, high-biomass summer cover crops to shade out nutsedge.
- Avoid overwatering and overfertilizing—rotate nutsedge-infested fields to crops that do not require high soil moisture levels or high levels of readily available N.
- Remedy soil drainage problems—chisel plow to break hardpan if necessary, grow deep rooted cover crops, and build soil organic matter.
- Avoid synthetic mulches when moderate to high populations of yellow nutsedge are present.
Consider allelopathy from crops like rye and sweet potato, and soil solarization, as additional "little hammers" against yellow nutsedge, but do not rely on these tactics alone to control the weed.
Consider using livestock to reduce a heavy infestation. The tubers have a mild, sweet flavor that is especially attractive to pigs, which have been reported to consume most of the tubers in an infested area within a few days (California Department of Food and Agriculture). Poultry or weeder geese can also be effective. For food safety and to comply with USDA Organic Standards, be sure to remove livestock from the field and incorporate droppings into the soil at least 120 days prior to expected harvest of the next food crop. A good time to run livestock to clean up a weedy field is after a cash crop harvest and just before planting a cover crop.
Finally, farmers in the southern U.S. should obtain a definitive species identification of their nutsedge populations. Yellow and purple nutsedges often occur together in the South. Although they have similar life cycles, their responses to climate, environmental conditions, and control tactics can differ significantly; for example, purple nutsedge becomes far more aggressive in hot climates. Determine whether yellow, purple, or both nutsedges are present in order to fine-tune your management strategy.
References Cited
- Bryson, C. T., and M. S. DeFelice. 2009. Weeds of the South. University of Georgia Press, Athens, GA.
- California Department of Food and Agriculture. 2012. Cyperus genus. State of California. (Available online at: http://www.cdfa.ca.gov/plant/IPC/encycloweedia/weedinfo/cyperus.htm) (verified 10 Sept 2012).
- Chase, C. A., T. R. Sinclair, D. G. Shilling, J. P. Gilreath, and S. J. Locascio. 1998. Light effects on rhizome morphogenesis in nutsedges (Cyperus spp): implications for control by soil solarization. Weed Science 46: 575–580. (Available online at: http://www.jstor.org/stable/4045964) (verified 10 Sept 2012).
- Drost, D. C., and J. D. Doll. 1980. The allelopathic effect of yellow nutsedge (Cyperus esculentus) on corn (Zea mays) and soybeans (Glycine max). Weed Science 28: 229–233. (Available online at: http://www.jstor.org/stable/4042988) (verified 10 Sept 2012).
- Harrison, H. F., Jr., and J. K. Peterson. 1991. Evidence that sweet potato (Ipomoea batatas) is allelopathic to yellow nutsedge (Cyperus esculentus). Weed Science 39: 308–312. (Available online at: http://www.jstor.org/stable/4044934) (verified 10 Sept 2012).
- Holm, L. G., D. L. Plucknett, J. V. Pancho, and J. P. Herberger, 1991. The world's worst weeds. Kriegar Publishing Company, Malabar, FL.
- Horak, M. J., J. S. Holt, and N. C. Ellstrand. 1987. Genetic variation in yellow nutsedge (Cyperus esculentus). Weed Science 35: 506-512. (Available online at: http://www.jstor.org/stable/4044520) (verified 10 Sept 2012).
- Jordan-Molero, J. E., and E. W. Stoller. 1978. Seasonal development of yellow and purple nutsedges (Cyperus esculentus and C. rotundus) in Illinois. Weed Science 26: 614–618. (Available online at: http://www.jstor.org/stable/4042940) (verified 10 Sept 2012).
- Keeley, P. E., and R. J. Thullen. 1978. Light requirements of yellow nutsedge (Cyperus esculentus) and light interception by crops. Weed Science 26: 10–16. (Available online at: http://www.jstor.org/stable/4042682) (verified 10 Sept 2012).
- Lapham, J., and D.S.H. Drennan. 1990. The fate of yellow nutsedge (Cyperus esculentus) seed and seedlings in soil. Weed Science 38: 125–128. (Available online at: http://www.jstor.org/stable/4045039) (verified 10 Sept 2012).
- Mohler, C. A., and A. DiTommaso. Unpublished. Manage weeds on your farm: a Guide to ecological strategies. Department of Crop and Soil Sciences, Cornell University. Pre-publication draft, version 5.1 (2008). Publication through SARE expected in 2012.
- Morales-Payan, W. M. Stall, D. G. Shilling, J. A. Dusky, and T. A. Bewick. 1997. Influence of nitrogen on the interference of purple and yellow nutsedge (Cyperus rotundus and Cyperus esculentus) with tomato (Lycopersicon esculentum). HortScience 32: 431. (Available online at: https://journals.ashs.org/hortsci/abstract/journals/hortsci/32/3/article-p431C.xml) (verified 14 Oct 2019).
- Mulligan, G. A., and B. E. Junkins. 1976. The biology of Canadian weeds. 17. Cyperus esculentus L. Canadian Journal of Plant Science 56: 339–350.
- Norsworthy, J. K., and J. T. Meehan, IV. 2005a. Wild radish-amended soil effects on yellow nutsedge (Cyperus esculentus) interference with tomato and bell pepper. Weed Science 53: 77–83. (Available online at: http://dx.doi.org/10.1614/WS-04-074R) (verified 10 Sept 2012).
- Norsworthy, J. K., and J. T. Meehan, IV. 2005b. Use of isothiocyanates for suppression of Palmer amaranth (Amaranthus palmeri), pitted morningglory (Ipomoea lacunose), and yellow nutsedge (Cyperus esculentus). Weed science 53: 884–890. (Available online at: http://dx.doi.org/10.1614/WS-05-056R.1) (verified 10 Sept 2012).
- Cardina, J., C. Herms, T. Koch, and T. Webster. 2012. Yellow Nutsedge, Cyperus esculentus. Ohio Perennial and Biennial Weed Guide. The Ohio State University. (Available online at: http://www.oardc.ohio-state.edu/weedguide/singlerecord.asp?id=150) (verified ).
- Peterson, J. K., and H. F. Harrison. 1995. Sweet potato allelopathic substance inhibits growth of purple nutsedge. Weed Technology 9: 277–280. (Available online at: http://www.jstor.org/stable/3987745) (verified 10 Sept 2012).
- Ransom, C. V., C. A. Rice, and C. C. Shock. 2009. Yellow nutsedge (Cyperus esculentus) growth and reproduction in response to nitrogen and irrigation. Weed Science 57: 21–25. (Available online at: http://dx.doi.org/10.1614/WS-08-080.1) (verified 10 Sept 2012).
- Russ, K., and C. Burgess. 2009. Nutsedge. Clemson University Extension. 5 pp. (Available online at: http://www.clemson.edu/extension/hgic/pests/weeds/hgic2312.html) (verified 10 Sept 2012).
- Santos, B. M., J. P. Morales-Payan, W. M. Stall, T. A. Bewick, and D. G. Shilling. 1997. Effects of shading on the growth of nutsedges (Cyperus spp.). Weed Science 45: 670–673. (Available online at: http://www.jstor.org/stable/4045892) (verified 10 Sept 2012).
- Stoller, E. W., D. P. Nema, and V. M. Bhan. 1972. Yellow nutsedge tuber germination and seedling development. Weed Science 20: 93–97. (Available online at: http://www.jstor.org/stable/4042040) (verified 10 Sept 2012).
- Stoller, E. W., and L. M. Wax. 1973. Yellow nutsedge shoot emergence and tuber longevity. Weed Science 21: 76–81. (Available online at: http://www.jstor.org/stable/4042258) (verified 10 Sept 2012).
- Tayyar, R. I., J.H.T. Nguyen, and J. S. Holt. 2003. Genetic and morphological analysis of two novel nutsedge biotypes from California. Weed Science 51: 731–739. (Available online at: http://dx.doi.org/10.1614/P2002-131) (verified 10 Sept 2012).
- Tumbleson, M. E., and T. Kommedahl. 1961. Reproductive potential of Cyperus esculentus by tubers. Weeds 9: 646–653.
- Uva, R. H., J. C. Neal, and J. M. DiTomaso, 1997. Weeds of the Northeast. Cornell University Press, Ithaca, NY.
- Wang, G., M. E. McGiffen, Jr., and E. J. Ogbuchiekwe. 2008. Crop rotation effects on Cyperus rotundus and C. esculentus population dynamics in southern California vegetable production. Weed Research 48: 420–428. (Available online at: http://dx.doi.org/10.1111/j.1365-3180.2008.00649.x) (verified 10 Sept 2012).
- Wang, G., M. E. McGiffen, Jr., E. J. Ogbuchiekwe, and L. Butler. 2009. Economic return of purple and yellow nutsedge management in vegetable production of southern California. Crop Protection 28: 319–326. (Available online at: http://dx.doi.org/10.1016/j.cropro.2008.11.011) (verified 10 Sept 2012).
- Webster, T. M. 2003. High temperatures and durations of exposure reduce nutsedge (Cyperus spp.) tuber viability. Weed Science 51: 1010–1015. (Available online at: http://dx.doi.org/10.1614/WS-03-018R) (verified 10 Sept 2012).
- Webster, T. M. 2005a. Mulch type affects growth and tuber production of yellow nutsedge (Cyperus esculentus) and purple nutsedge (Cyperus rotundus). Weed Science 53: 834–838. (Available online at: http://dx.doi.org/10.1614/WS-05-029R.1) (verified 10 Sept 2012).
- Webster, T. M. 2005b. Patch expansion of purple nutsedge (Cyperus rotundus) and yellow nutsedge (Cyperus exculentus) with and without polyethylene mulch. Weed Science 53: 839–845. (Available online at: http://dx.doi.org/10.1614/WS-05-045R.1) (verified 10 Sept 2012).
- Webster, T. M. 2006. Weed survey – southern states. Vegetable, fruit and nut crops subsection. Proceedings of the Southern Weed Science Society 59: 260–277. (Available online at: http://www.swss.ws/wp-content/uploads/docs/2006%20Proceedings-SWSS.pdf) (verified 10 Sept 2012).



