eOrganic author:
Dr. Mark Schonbeck, Virginia Association for Biological Farming
Abstract
Yellow nutsedge and purple nutsedge are grass-like perennial weeds that can cause severe losses in vegetable and row crops. Nutsedges grow during the frost-free season, spreading and propagating through an extensive underground network of rhizomes, bulbs, and small, starchy tubers or “nutlets.” Within a single growing season, one tuber can give rise to hundreds of shoots in a dense patch 3–6 feet across, and form over 1,000 new tubers.
Yellow nutsedge has bright green to yellow-green foliage, straw-colored or golden-yellow flower heads, and tubers borne singly at the tips of short rhizomes. It occurs throughout the United States and into southern Canada, and is most troublesome in moist or irrigated soils.
Purple nutsedge has dark green foliage, purple to red-brown flower heads, and tubers borne in chains along rhizomes. It is most troublesome in tropical and warm–temperate climates, including the southern United States and California.
Nutsedges are difficult to manage because of their large underground reserves, tolerance to cultivation and environmental stresses, and ability to penetrate mulches. To reduce heavy infestations, till when shoots first emerge in late spring, and repeat tillage to sever re-emerging shoots before they have 6 leaves. Soil solarization during summer can complement tillage in reducing the nutsedge population. Follow tillage or solarization with an aggressive summer cover crop to suppress nutsedge regrowth and replenish soil organic matter consumed by tillage.
To prevent light nutsedge infestations from worsening:
- Time tillage and cultivation to disrupt nutsedge growth (late spring–early summer) and tuber formation (late summer).
- Use tillage implements that bring rhizomes and tubers to the surface to dry out or freeze.
- Design crop rotation to disrupt the nutsedge life cycle and facilitate timely tillage.
- Grow competitive, shading crops during the frost-free season, such as bush bean, sweet potato, and summer cover crops.
- Manage nutrients and moisture to favor the crop over nutsedge.
- Run swine, poultry, or weeder geese in the field after crop harvest to consume nutsedge.
Introduction
The nutsedges are grass-like, colony-forming, perennial weeds that grow actively during the frost-free season, spread by rhizomes, and propagate from year to year by small, starchy tubers (sometimes called “nutlets," which give the weeds their common name). Nutsedges, also called nutgrasses, are difficult to manage because of their tolerance to heat, drought, and flooding; their prolific underground vegetative reproduction; and their ability to regrow after cultivation and to penetrate most mulches.
Purple nutsedge causes serious losses to a wide range of crops in tropical, subtropical and warm–temperate regions—including the southern United States and California—and has been cited as the world's most costly agricultural weed (Holm et al., 1991). Yellow nutsedge occurs throughout the United States and into Canada, and is a major weed in the Northeast (Else, 1996) and in irrigated crops in the Northwest (Ransom et al., 2009). The two species often occur together and are considered major weeds of vegetable production in the South (Webster, 2006).
Description and Identification
Nutsedges are grass-like plants in the sedge family (Cyperaceae) with narrow, linear, folded leaves 4–12 inches long by 0.1–0.4 inch wide. Nutsedges can be distinguished from true grasses by the 3-ranked arrangement of leaves (Fig. 1a), a simple leaf blade with no sheath or collar, and solid stems triangular in cross section. Grasses have round stems that are hollow in the internodes, and leaves in a 2-ranked arrangement showing distinct sheath, collar, and blade regions (Fig. 1b).


Figure 1. (a) Yellow nutsedge, showing the characteristic 3-ranked leaf arrangement of the sedge family (Cyperaceae). (b) Weedy grasses with the 2-ranked leaf arrangement of true grasses (Poaceae). Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Individual flowers are small, inconspicuous, and borne in a cluster of spikes radiating 0.5–2 inches out from the top of the flower stalk, and subtended by a whorl of narrow, leaflike bracts (Fig. 2). Each flower forms a single-seeded dry fruit (achene) similar to grasses.


Figure 2. (a) Yellow nutsedge flowers. (b) Purple nutsedge flowers. Photo credits: (a) John W. Everest, Auburn University, Bugwood.org. (b) James A. Miller, USDA Forest Service, Bugwood.org.
Nutsedges form complex underground networks of vegetative structures (Fig. 3), including basal bulbs from which the shoots arise; thin, wiry rhizomes; tubers; and a fibrous root system extending up to 4 feet deep. Tubers (nutlets) are about 0.4–0.8 inch long, scaly, whitish and succulent at first, turning firm and brown–black as they mature. The mature tuber is packed with starch and has 40–50% dry matter content (Holm et al., 1991).
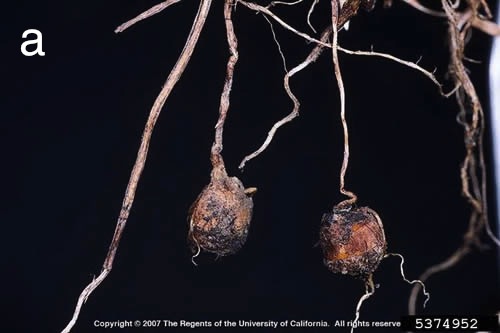

Figure 3. (a) Yellow nutsedge tubers. (b) A chain of 4 purple nutsedge plants, consisting of a mother plant and two generations of daughter plants connected by rhizomes. Each plant arises from a small basal bulb. Photo credits: (a) Joseph M. DiTommaso, University of California-Davis, Bugwood.org. (b) Mark Schonbeck, Virginia Association for Biological Farming.
Yellow nutsedge is distinguished by its bright "spring-green" foliage (Fig. 4a); yellow, straw-colored, or golden inflorescence (Fig. 2a); and tubers borne singly at the ends of rhizomes. Purple nutsedge foliage is a darker green (Fig. 4b), the inflorescence is purplish to reddish-brown (Fig. 2b), and tubers are borne in chains, with individual tubers set 2–10 inches apart along the rhizome.


Figure 4. (a) Bright green foliage of yellow nutsedge. (b) Darker green foliage of purple nutsedge. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Life Cycle and Reproduction
Nutsedges reproduce primarily by tubers. In temperate regions, new growth begins after the spring frost date. A rhizome emerges from the tuber, grows toward the soil surface, and forms a basal bulb from which the shoot and fibrous roots emerge. Basal bulbs usually form within 3 inches of the soil surface, although purple nutsedge bulbs have been found at 4–8 inches (Hauser, 1962; Fig. 5). After several weeks' shoot growth, new rhizomes grow laterally from basal bulbs, giving rise to new basal bulbs and shoots (daughter plants). The process continues for 2–4 months, during which a single overwintered tuber can give rise to a patch several feet across.

Figure 5. In this purple nutsedge infestation, most of the basal bulbs were found within 2 inches of the soil surface, with a few as deep as 4 inches. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Nutsedges generally bloom and begin to form new tubers about 7–8 weeks after initial shoot emergence. In temperate latitudes, tuber formation is triggered by shortening daylength in late summer and accelerates while aboveground growth rates decline (Hauser, 1962; Jordan-Molero and Stoller, 1978). Tubers develop throughout the top 6–10 inches of soil, and a few may occur at 12–18 inches (Tumbleson and Kommedahl, 1961; Holm et al., 1991). Foliage dies back with the first fall frost, and the weed overwinters as dormant tubers.
Yellow nutsedge reproduces most prolifically in cool–temperate climates. One yellow nutsedge tuber has given rise to 1,900 shoots and 6,900 new tubers within 1 year in Minnesota (Tumbleson and Kommedahl, 1961), and to 1,700–3,000 shoots and 19,000–20,000 tubers within 4 months in irrigated fields in Oregon (Ransom et al., 2009). Purple nutsedge multiplied several times faster than yellow nutsedge in a field trial in Georgia (Webster, 2005b), but the reverse was true in an outdoor container trial in Illinois (Jordan-Molero and Stoller, 1978).
Viable seeds are occasionally formed through cross pollination, especially in yellow nutsedge; however, seeds are not considered important in propagation of either weed (Holm et al., 1991).
Tuber Dormancy, Germination, and Dispersal
Newly mature nutsedge tubers are dormant. In yellow nutsedge, dormancy is broken over winter by chilling temperatures of 35–50°F (Holm et al., 1991; Stoller and Wax, 1973). Diurnally fluctuating temperatures with maxima near 85–95°F break tuber dormancy in purple nutsedge (Miles et al., 1996). Apical dominance regulates tuber sprouting in purple nutsedge tuber chains, so that the terminal tuber sprouts while the others remain dormant (Kawabata and Nishimoto, 2003). Individual tubers of either species can remain viable in the soil for several years.
Nutsedge tubers are spread to new locations in soil clinging to farm equipment and tires, and sometimes by animals or flood waters. In addition, tubers can occur in the soil or growing medium of potted plants, in compost and mulch, or as a contaminant in crop harvests.
Growth Habit, Environmental Tolerances, and Impact on Crops
Nutsedges have the C4 photosynthetic pathway, and thus grow rapidly in high temperature and high light conditions. Although the plants are low-growing (4–30 inches), they form dense stands that carpet the ground (Fig. 6), and can develop tremendous underground biomass. Heavy infestations can attain 4–9 tons dry weight per acre in the tubers alone (Tumbleson and Kommedahl, 1961; Holm et al., 1991). Large energy reserves in the tubers allow nutsedge to survive long periods of shade, drought, and flooding, and to tolerate repeated shoot removal by mowing or cultivation (Santos et al., 1997; Stoller and Wax, 1973).

Figure 6. A carpet of yellow nutsedge covers the ground in a home garden in Virginia. Photo Credit: Mark Schonbeck, Virginia Association for Biological Farming.
Nutsedge thrives under a wide range of conditions, although some species differences exist (Table 1). Yellow nutsedge is slightly less heat tolerant than purple nutsedge (Webster, 2003), but more tolerant to flooding, cold, and light shade. A heavy crop canopy suppresses nutsedge growth and tuber set (Santos et al., 1997; Jordan-Molero and Stoller, 1978), but existing tubers remain viable and sprout when the shade is removed (Holm et al., 1991). Tubers can be killed by heating to 122°F for 10–24 hours (Webster 2003), or desiccation to 15–24% moisture content (Holm et al., 1991). However, tubers located deeper than 2 inches in the soil are generally protected from lethal temperatures and dehydration.
Nutsedges often first appear in wet areas and may indicate poor drainage or overirrigation (Fig. 7); however, once established, the weed can persist in normal to dry conditions (Russ and Burgess, 2009). Yet, some farmers who use organic inputs and judicious tillage to improve soil tilth and drainage have observed a decrease in yellow nutsedge populations as soil quality improves (Josh Hardin, farmer in Arkansas, pers. commun.; Charles Maloney, farmer in Virginia, pers. commun.).

Figure 7. Yellow nutsedge growing vigorously in wet, poorly drained soil with low organic matter. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Nutsedges compete aggressively against warm-weather crops for moisture and nutrients (Fig. 8). Cool-season crops planted in fall escape nutsedge competition, as the weed enters dormancy at this time of year (McGiffen et al., 1997). In tropical regions, purple nutsedge can inflict severe losses on crops many times its height, including sugarcane and coffee (Holm et al., 1991). In field trials in the low desert of California, purple nutsedge was very hard to control with cultivation and crop rotation—causing 80-95% crop losses in winter broccoli—while yellow nutsedge had little effect on broccoli yield (Wang et al., 2008). Purple nutsedge is less aggressive near the northern edge of its range (e.g., North Carolina), where seasonal cold limits its growth. Yellow nutsedge is most troublesome in temperate, high-moisture soils, and in irrigated crops maintained at a high water potential, such as onion or rice (Ransom et al., 2009).

Figure 8. Severe infestation of purple nutsedge in cotton. Photo credit: Charles T. Bryson, USDA Agricultural Research Service, Bugwood.org.
Nutsedges also affect crops by releasing allelopathic (growth-inhibiting) substances (Drost and Droll, 1980; Friedman and Horowitz, 1971); by hosting root-knot nematodes (Schroeder et al., 1997); and by damaging root crops as rhizomes pierce the edible portion. A heavy infestation of yellow nutsedge in potato can render every potato in the field unmarketable (Holm et al., 1991).
In Oregon field trials, yellow nutsedge responded only slightly to nitrogen (Ransom et al., 2009), and good soil fertility apparently increased crop vigor relative to this weed in the northeastern United States (Mohler and DiTommaso, unpublished). In the southern United States, purple and yellow nutsedge compete more aggressively against vegetable crops at higher fertilizer N rates (Morales-Payan et al., 1997; Santos et al., 1998). High soil moisture levels have been reported to intensify purple nutsedge competition against cotton (Cinco-Castro and McCloskey, 1997) and to stimulate yellow nutsedge growth and propagation (Ransom et al., 2009).
Shading by crops such as bush snap bean, potato, sweet potato, and sweet corn can curb nutsedge growth and tuber set, and crop competition has been cited as a nutsedge management tactic (Keeley and Thullen, 1978; McGiffen et al., 1997; Neeser et al., 1997; William, 1976; William and Warren, 1975). A few crops may retard nutsedge growth by allelopathy, including sorghum (Cheema et al., 2004), rye (Mohler and DiTommaso, unpublished), and sweet potato (Neeser et al., 1997).
The sharp, pointed shoots of nutsedge readily emerge through organic mulches, and can puncture opaque plastic mulches (Webster, 2005a) (Fig. 9). Black plastic slowed the spread of yellow nutsedge by about 50% in field trials in Georgia, but doubled the spread of purple nutsedge, which responded to soil warming under the film (Webster, 2005b). However, in the low desert of southeastern California, a black plastic film sufficiently raised temperatures throughout the top 6 inches of the soil profile to kill nutsedge tubers and rhizomes, thus providing a substantial measure of control (Wang et al., 2008). Nutsedge shoots emerging under clear or translucent films become trapped because their leaves open in response to light before the growing point can puncture the film (Patterson, 1998). The trapped foliage is often heat-killed, whereas tubers are weakened but rarely killed.

Figure 9. Nutsedge has penetrated the plastic film mulch and is interfering with the pepper crop. Photo credit: Theodore Webster, Agricultural Research Service, Bugwood.org.
| Yellow Nutsedge | Purple Nutsedge | |
|---|---|---|
| TOP GROWTH | Yellow-green to bright green; 8–24 inches (36 maximum) | Dark green; 4–18 inches (30 maximum) |
| FLOWER HEAD | Yellow, gold, or straw-colored | Purplish to reddish-brown |
| SEEDS | Viable if outcrossed | Mostly non-viable |
| TUBERS | Borne singly at ends of rhizomes; edible, pleasant flavor (chuffa) | Borne in chains on rhizomes; bitter and hard |
| TUBER DORMANCY | Broken by chilling (35-50°F) over winter | Broken by heat (95°F), fluctuating temperatures, or release of apical dominance when tuber chains break |
| RANGE IN NORTH AMERICA | Throughout United States into Canada | Mostly southern half of United States |
| MOST AGGRESSIVE REGIONS | Temperate region, irrigated fields | Tropical or subtropical regions |
| ABILITY TO PENETRATE ORGANIC MULCH | Very high | Very high |
| ABILITY TO PENETRATE BLACK PLASTIC | Moderate (mulch slows spread) | Very high (mulch stimulates spread) |
| ABILITY TO PENETRATE CLEAR PLASTIC | Low (leaves open and are trapped) | Low (leaves open and are trapped) |
| DROUGHT TOLERANCE | Moderate to high | Very high |
| HEAT TOLERANCE | High | Very high |
| FLOODING TOLERANCE | Very high (thrives in wet soil) | High (goes dormant) |
| COLD TOLERANCE | Moderate (tubers ~20°F) | Low (all parts frost tender) |
| SHADE TOLERANCE | Low–moderate | Low (shoots die back, tubers persist) |
| NUTRIENT RESPONSE | Variable (low in cool climates) | High (more aggressive at high N) |
Nutsedge populations show significant variation across their geographic range (Holm et al., 1991), and unusually robust strains of both species have been reported in California (Tayyar et al., 2003) and Brazil (William, 1976). This suggests that nutsedges can undergo genetic adaptation to climate, soil, or production practices—possibly through occasional sexual reproduction, genetic recombination, and selection.
Management
Organic growers have been advised to avoid fields infested with nutsedge (Bangarwa et al., 2008). However, in the real world, organic vegetable growers must often cope with these weeds. To maximize chances of doing so successfully, growers should:
- Identify the nutsedge species, learn its strengths and vulnerabilities (Table 1), and plan accordingly.
- Use an integrated strategy that combines multiple preventive and control measures.
- Bring heavy infestations under control before attempting organic production, especially summer vegetables and perennial crops.
- Be vigilant and proactive to keep small populations from exploding.
Prevention and Early Detection
If nutsedge is present in your region but not yet on your farm, avoid importing it! Ensure that nursery stock, topsoil, compost, manure, and mulch materials are free from nutsedge plants or tubers before buying them. Thoroughly clean borrowed or rented field equipment before bringing it onto the farm. If just one or two fields on the farm have nutsedge, avoid spreading it to other fields by carefully cleaning tools, equipment, vehicle tires, and footwear immediately after working infested fields.
Nutsedge outbreaks often begin in poorly drained, compacted areas within crop fields. Improve drainage and soil quality in such areas by subsoiling, cover cropping (include "biodrilling" cover crops like tillage radish, sorghum-sudangrass, and sweetclover), adding organic matter, and adopting conservation tillage and controlled traffic practices to minimize compaction.
Monitor fields regularly and act promptly at first sighting of nutsedge. Small, local infestations can be eradicated by digging out all plants, rhizomes, and tubers. Dig at least 10 inches deep and 10 inches beyond the perimeter of the patch (Russ and Burgess, 2009).
Tillage and Cultivation
Timely repeated tillage is the main organic strategy for reducing a heavy nutsedge infestation. Begin tilling in late spring after shoots have emerged but before they can form new rhizomes or daughter plants. Till 6–8 inches deep to break tuber dormancy. Deplete rhizomes and tubers by tilling or cultivating again whenever emerging shoots have at least 3 but not more than 6 leaves (Mohler and DiTommaso, unpublished; Russ and Burgess, 2009); this may require cultivating every 2–3 weeks (Fig. 10). If practical, work deep enough to sever basal bulbs from their root systems (William, 1976).

Figure 10. Purple nutsedge regrowth photographed 13 days after initial tillage. Cultivate now. The small shoots on the right have drawn down underground reserves to their low point, whereas the larger plant to the left has already begun to rebuild below-ground biomass. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Be aware that tillage, especially rotary tillage, can spread a localized infestation by moving tubers around in the soil. This is particularly true when the soil is wet and liable to cling to tines or plowshares, thereby facilitating long-distance transport of tubers. Whenever practical, till or cultivate for nutsedge control when the soil is dry enough to leave implement tines clean (Russ and Burgess, 2009). To be safe, powerwash or otherwise clean the implement before leaving an infested field.
Use a field cultivator or similar implement to bring rhizomes and tubers to the surface, thereby exposing them to desiccation or freezing. Several passes during dry weather can significantly reduce the population (Tumbleson and Kommedahl, 1961; Mohler and DiTommaso, unpublished).
Control nutsedge that emerges in crops by cultivating whenever emerging weeds have 3–6 leaves. An economic analysis of nutsedge control strategies for vegetable production in southern California showed net returns for multiple cultivations > summer solarization > spring and summer smother crops (Wang et al., 2009). Till promptly after a spring vegetable harvest or immediately prior to a midsummer vegetable planting to knock the weed back before it can propagate.
Soil solarization
In very hot and sunny climates such as the desert southwest region of the United States, soil solarization during late June to early August (when solar radiation and temperatures are near maximum) can significantly reduce a nutsedge infestation. In Florida, solarization with clear plastic does not kill most purple nutsedge tubers; however, it causes daily pulses of high temperature that break tuber dormancy. Tubers are gradually weakened when emerging shoots open under the clear film, become trapped, and are heat-killed (Chase et al., 1999). Because tuber dormancy is a major factor in nutsedge persistence, solarization can be an important component of the control strategy.
Till or cultivate to remove existing nutsedge top growth, stimulate additional tuber sprouting, and prepare a smooth surface. Lay clear or translucent plastic film, and leave in place for 3–6 weeks during late June through early August when solar radiation and air temperatures are near their maximum. To ensure effective heating of the soil profile, be sure the soil is moist—irrigate before laying plastic if necessary—and the film fits tightly and evenly over the soil surface. After solarization, cultivate to remove emerging nutsedge regrowth when it reaches the 3–6 leaf stage.
Crop Competition, Crop Rotation, and Cover Crops
Exploit the two weaknesses of nutsedges: their short stature and shade intolerance. Design crop rotations and production systems to maximize crop competition against weeds during the frost-free growing season. Choose vigorous, adapted varieties of heavy-canopy crops like potato, bush bean, and sweet potato; and tall crops like sweet corn or trellised tomato. Canadian potato growers have found that growing a potato variety with particularly heavy foliage (‘Kennebec’) can substantially reduce yellow nutsedge populations in the following season, compared to fields with less-competitive varieties (‘Katahdan’ or ‘Irish Cobbler’) (Mulligan and Junkins, 1976).
Choose optimum planting dates so that crops are not subjected to growth-retarding cold or other stresses. Use crop row spacing and orientation that promotes canopy closure (except for crops in which this would increase the risk of fungal diseases), or effective shading of alleys.
Design crop rotation and select planting dates so that the late spring flush of nutsedge either emerges beneath a dense crop canopy (such as a winter or spring cover crop like rye/vetch, or oats /field peas), or can be knocked out by tillage after harvest of an early spring vegetable like spinach or salad greens. Similarly, disrupt the onset of tuber production in late summer, either by providing heavy shade (such as snap bean or sweet potato at full vegetative growth), or through tillage after a midsummer vegetable harvest.
Follow spring vegetables with an aggressive, fast-growing, summer cover crop that will shade out emerging nutsedge. Use optimal seeding rates and methods to maximize cover crop weed suppression. Buckwheat closes canopy rapidly and can provide excellent weed suppression during brief (30–50 day) fallow periods in frost-free, moderately warm weather. For longer fallow or hotter conditions, combine a tall grass (sudangrass, sorghum-sudangrass hybrid, or pearl millet) with a heavy-canopy summer legume (cowpea, forage soybean, velvetbean, or lablab bean) for maximum competition. In fields with nutsedge populations too heavy to allow successful production of summer vegetables, grow high biomass summer cover crops and follow with cool-season vegetables in fall, winter, or early spring, when nutsedge is dormant.
No-till methods of terminating cover crops can enhance soil quality and suppress annual weeds; however, this technique should not be attempted until nutsedge has been eliminated from the field.
The allelopathic potential of rye, sorghum-sudangrass, and sweet potato may contribute to nutsedge suppression and should be considered in designing the rotation. However, do not rely on allelopathy alone to control these weeds.
Soil, Nutrient, and Moisture Management
Use slow-release organic fertilizers and in-row drip irrigation to deliver moisture and nutrients preferentially to the crop. Sidedress faster-release N materials to heavy feeders when the crops are established and entering their rapid growth phase. Avoid overapplying N and overwatering. Rotate crops that require frequent irrigation to maintain high soil water potential with crops that thrive in drier conditions.
Pest Management – Nematodes
If root-knot nematodes or other pest nematodes for which nutsedge can be a host are present, it is especially important to reduce nutsedge populations to low levels before attempting production of susceptible crops. Nutsedges do not suffer ill effects from the nematode (Schroeder et al., 1997), and the two together put crops in double jeopardy. Building soil organic matter and soil life diversity can help combat pest nematodes.
Nutsedge Removal by Livestock and Poultry
Consider using swine, hens, or weeder geese to consume nutsedge. Laying hens confined in small fenced areas (up to 50 ft by 50 ft for one chicken coop or chicken tractor) at densities equivalent to 480 birds per acre have been reported to clean up a heavy nutsedge infestation in one season. Weeder geese at 8 to 16 birds per acre have significantly reduced nutsedge competition in cotton (Mayton et al., 1945). Swine will root out and consume nutsedge tubers (Ohio State University), and 60-75 hogs have been reported to remove purple nutsedge tubers from a 2.5 acre field in one day in India (Open Source for Weed Assessment in Lowland Paddy Fields).
Remember that USDA certified organic production requires at least a 120-day interval between incorporation of livestock or poultry manure and harvest of any food crops that might have exposure to soil or soil splash; and a 90-day interval for food crops not exposed to soil, such as tree fruit and sweet corn. Remove livestock, poultry, or geese from the field and incorporate droppings at the suitable time prior to organic food crop production to protect food safety and meet USDA Organic requirements. A good time to use livestock or poultry for nutsedge control is immediately after cash crop harvest and before planting a cover crop.
Mowing
Mowing is less effective against nutsedge than tillage and cultivation, yet it may be warranted where tillage is not practical, such as in alleys between trellised tomato rows. Cut top growth as close to the ground as possible with a push mower or weed whacker. Mow before tuber set (mid-late summer), and repeat as needed to keep the weed from propagating.
Mulching
Mulching is generally ineffective against nutsedge. Do not use opaque synthetic mulch in fields with moderate to high nutsedge populations. In addition to causing yield losses, nutsedge growing through plastic film or landscape fabrics can complicate end-of-season plastic removal.
Multiple, Integrated Tactics
None of the above measures alone will bring a significant nutsedge problem under control. Use a multi-component, integrated strategy. For example, follow an early vegetable harvest with one or more tillage passes to disrupt emerging nutsedge, solarize in June or July, then follow with an aggressive summer cover crop. When nutsedge populations decline sufficiently to allow summer vegetable production, combine vigorous, locally-adapted varieties with best management of water, nutrients, and pest nematodes, as well as timely cultivation to maximize the crop's advantage over nutsedge.
References and Citations
- Bangarwa, S. K., J. K. Norsworthy, P. Jha, and M. Malik. 2008. Purple nutsedge (Cyperus rotundus) management in an organic production system. Weed Science 56: 606–613.
- Chase, C. A., T. R. Sinclair, and S. J. Locascio. 1999. Effects of soil temperature and tuber depth on Cyperus spp. control. Weed Science 47: 467-472.
- Cheema, Z. A., A. Khaliq, and S. Saeed. 2004. Weed control in maize (Zea mays L.) through sorghum allelopathy. Journal of Sustainable Agriculture 23: 73–87.
- Cinco-Castro, R., and W. B. McCloskey. 1997. Purple nutsedge (Cyperus rotundus L.) competition with cotton under wet and dry soil moistures. Weed Science Society of America Abstracts 37: 55.
- Drost, D. C., and J. D. Droll. 1980. The allelopathic effect of yellow nutsedge (Cyperus esculentus) on corn (Zea mays) and soybean (Glycine max). Weed Science 28: 227-233.
- Else, M. J. 1996. Preventing weed problems: five weeds to watch out for. The Natural Farmer 2(29) (Summer, 1996): 10–11.
- Friedman, T., and M. Horowitz. 1971. Biologically active substances in subterranean parts of purple nutsedge. Weed Science 19:
- Hauser, E. W. 1962. Development of purple nutsedge under field conditions. Weeds 10: 315-321.
- Holm, L. G., D. L. Plucknett, J. V. Pancho, and J. P. Herberger. 1991. The world's worst weeds. Kriegar Publishing Company, Malabar, FL.
- Jordan-Molero, J. E., and E. W. Stoller. 1978. Seasonal development of yellow and purple nutsedges (Cyperus esculentus and C. rotundus) in Illinois. Weed Science 26: 614–618.
- Kawabata, O., and R. K. Nishimoto. 2003. Temperature and rhizome chain effect on sprouting of purple nutsedge (Cyperus rotundus) ecotypes. Weed Science 51: 348–355.
- Keeley, P. E., and R. J. Thullen. 1978. Light requirements of yellow nutsedge (Cyperus esculentus) and light interception by crops. Weed Science 26: 10–16.
- Mayton, E. L., E. V. Smith, and D. King. 1945. Nutgrass eradication studies IV: use of chickens and geese in the control of nutgrass, Cyperus rotundus L. Journal of the American Society of Agronomy 37(10): 785-791.
- McGiffen, M. E., Jr., D. W. Cudney, E. J. Obguchiekwe, A. Baameur, and R. L. Kallenbach. 1997. Management alternatives for purple and yellow nutsedge (Cyperus rotundus and C. esculentus). HortScience 32: 431.
- Miles, J. E., R. K. Nishimoto, and O. Kawabata. 1996. Diurnally alternating temperatures stimulate sprouting of purple nutsedge (Cyperus rotundus) tubers. Weed Science 44: 122-125.
- Mohler, C. A., and A. DiTommaso. Unpublished. Manage weeds on your farm: a guide to ecological strategies. Department of Crop and Soil Sciences, Cornell University. Pre-publication draft, version 5.1 (2008). Publication through SARE expected in 2012.
- Morales-Payan, J. P., W. M. Stall, D. G. Shilling, J. A. Dusky, and T. A. Bewick. 1997. Influence of nitrogen on the interference of purple and yellow nutsedge (Cyperus rotundus and Cyperus esculentus) with tomato (Lycopersicon esculentum). HortScience 32: 431.
- Mulligan, G. A., and B. E. Junkins. 1976. The biology of Canadian weeds 17. Cyperus esculentus L. Canadian Journal of Plant Science 56: 339-350.
- Neeser, C., R. Aguero, and C. J. Swanton. 1997. Incident photosynthetically active radiation as a basis for integrated management of purple nutsedge (Cyperus rotundus). Weed Science 45: 777–783.
- Ohio State University. Ohio perennial and biennial weed guide: Yellow Nutsedge Cyperus esculentus. (Available online at: https://www.oardc.ohio-state.edu/weedguide/single_weed.php?id=84)(verified 31 Dec 2019).
- Open Source for Weed Assessment in Lowland Paddy Fields. Cyperus rotundus L. - Cyperaceae - Monocotyledon. 3 pp. (Available online at: http://agritrop.cirad.fr/580508/)(verified 31 Dec 2019).
- Patterson, D. T. 1998. Suppression of purple nutsedge (Cyperus rotundus) with polyethylene film mulch. Weed Technology 12: 275–280.
- Ransom, C. V., C. A. Rice, and C.C. Shock. 2009. Yellow nutsedge (Cyperus esculentus) growth and reproduction in response to nitrogen and irrigation. Weed Science 57:21–25.
- Russ, K., and C. Burgess. 2009. Nutsedge. Clemson University Extension. 5 pp. (Available online at: www.clemson.edu/extension/hgic/pests/weeds/hgic2312.html)(verified 7 Jan 2013).
- Santos, B. M., J. P. Morales-Payan, W. M. Stall, and T. A. Bewick. 1997. Influence of tuber size and shoot removal on purple nutsedge (Cyperus rotundus) regrowth. Weed Science 45: 681-683.
- Santos, B. M., J. P. Morales-Payan, W. M. Stall, and T. A. Bewick. 1998. Influence of purple nutsedge (Cyperus rotundus) density and nitrogen rate on radish (Raphanus sativus) yield. Weed Science 46: 661–664.
- Schroeder, J., S. H. Thomas, and L. Murray. 1997. Yellow (Cyperus esculentus L.) and purple (C. rotundus L.) nutsedge response to root-knot nematodes. Weed Science Society of America Abstracts 37: 52.
- Stoller, E. W., and L. M. Wax. 1973. Yellow nutsedge shoot emergence and tuber longevity. Weed Science 21: 76–81.
- Tayyar, R. I., J. H. T. Nguyen, and J. S. Holt. 2003. Genetic and morphological analysis of two novel nutsedge biotypes from California. Weed Science 51: 731–739.
- Tumbleson, M. E., and T. Kommedahl. 1961. Reproductive potential of Cyperus esculentus by tubers. Weeds 9: 646–653.
- Wang, G., M. E. McGiffen, Jr., and E. J. Ogbuchiekwe. 2008. Crop rotation effects on Cyperus rotundus and C. esculentus population dynamics in southern California vegetable production. Weed Research 48 (5): 420-428.
- Wang, G., M. E. McGiffen, Jr., E. J. Ogbuchiekwe, and L. Butler. 2009. Economic return of purple and yellow nutsedge management in vegetable production of southern California. Crop Protection 28: 319-326.
- Webster, T. M. 2003. High temperatures and durations of exposure reduce nutsedge (Cyperus spp.) tuber viability. Weed Science 51: 1010–1015.
- Webster, T. M. 2005a. Mulch type affects growth and tuber production of yellow nutsedge (Cyperus esculentus) and purple nutsedge (Cyperus rotundus). Weed Science 53: 834–838.
- Webster, T. M. 2005b. Patch expansion of purple nutsedge (Cyperus rotundus) and yellow nutsedge (Cyperus esculentus) with and without polyethylene mulch. Weed Science 53: 839–845.
- Webster, T. M. 2006. Weed Survey – Southern States. Vegetable, fruit and nut crops subsection. Proceedings of the Southern Weed Science Society 59: 260–277.
- William, R. D. 1976. Purple nutsedge: tropical scourge. HortScience 11: 357–364.
- William, R. D., and G. F. Warren. 1975. Competition between purple nutsedge and vegetables. Weed Science 23: 317-323.



