eOrganic authors:
Laura Kaminsky, Pennsylvania State University
Mara Cloutier, Pennsylvania State University
Suzanne Fleishman, Pennsylvania State University
Sarah Isbell, Pennsylvania State University
Kristy Borrelli, Pennsylvania State University
Terrence Bell, Pennsylvania State University
Introduction: What is a Microbe?
A microbe, or microorganism, is a very small life form that we typically cannot see without the help of a microscope. Most of what we consider to be microbes can be classified as fungi, bacteria, archaea, protozoa, or viruses. Archaea are superficially similar to bacteria but are genetically and biochemically distinct. Additionally, tiny insects and nematodes that are not visible to the naked eye are sometimes included in definition of microorganisms, though they are many times larger than fungi or bacteria. Microbes can be found in all types of environments including human skin, animal guts, rainfall, caves, plants, and soils. It has been estimated that a single gram of soil can contain up to several billion bacteria.
In the same way that humans require food, microbes require an energy source to survive and grow their population in these environments, as well as carbon and other nutrients to build the molecules that make up their cells. Besides using carbon to build their cells, many microbes use carbon-based materials as their energy source as well. In soils, carbon sources available to microbes include plant litter, carbon compounds released by plant roots, and living or dead soil organisms including other microbes and larger soil fauna like worms and insects. Collectively, these carbon-based materials derived from living or once-living things are referred to as soil organic matter. Microbially-available carbon is called active soil organic matter, while unavailable carbon that has become bound to soil minerals or aggregates is called stable soil organic matter.
Where are Microbes Located in Farm Soils?
Microbes are not distributed evenly within soil environments, because their growth depends on the availability of water, carbon, and other nutrients. As an analogy, think of an oasis with a water source in an otherwise dry desert. Because of the availability of water, an ecosystem with far greater biomass and diversity can flourish in the oasis compared to surrounding desert areas. A plant root or piece of plant litter is like a microbial oasis, because it provides otherwise scarce carbon and energy sources to soil microbes. Through photosynthesis, plants convert carbon dioxide from the air into carbon-rich sugars and uses them to build other carbon-rich compounds and plant tissue. Microbes can then use these plant-derived carbon sources either when plant parts die or when carbon-based compounds are released or exude from plant roots. As such, microbial biomass and diversity tends to be much higher in the soil immediately surrounding plant roots—a zone known as the rhizosphere—and where there are other deposits of organic matter (e.g., at sites of decaying plant materials). Other areas of the soil are more like microbial deserts, where fewer microbes can survive.
Many soil microbes require oxygen as an input while breaking down carbon materials to generate energy—a process called aerobic respiration. Others thrive only in the absence of oxygen (such as in saturated, waterlogged soils), performing fermentation or other anaerobic processes to break down carbon materials instead of aerobic respiration. Some microbes are able to switch between these processes and thrive under either dry or saturated conditions. Well-drained soils with plenty of oxygen tend to support a greater activity and diversity of microbes because aerobic respiration is the more efficient microbial process for generating energy.
More microorganisms live closer to the soil surface due to the increased availability of oxygen and plant litter, the higher density of plant roots, and the addition of human-added carbon amendments like compost and mulch. Microbial abundance decreases with soil depth, as there are fewer carbon sources for microbes to consume and less oxygen to support aerobic respiration (Fig. 1). However, if plants with deeper roots are present, these deep rhizospheres also act as microbial oases of oxygen and carbon and can support microbial growth further down in the soil profile.
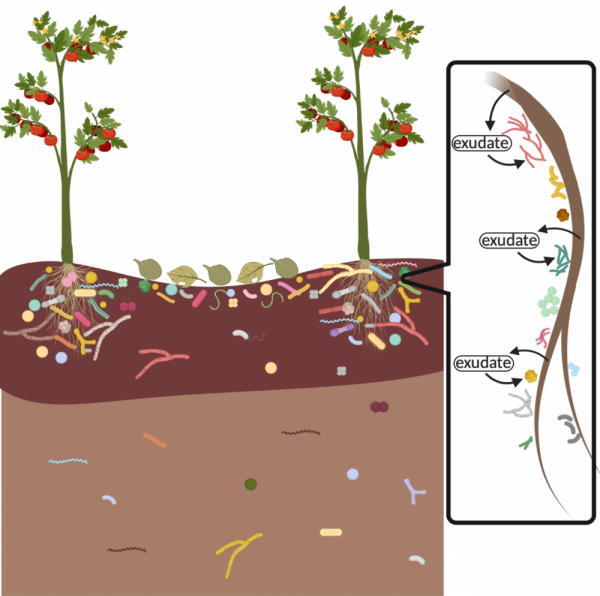
Figure 1: Microbial abundance in a soil profile. Microbes are concentrated at the soil surface, near plant roots and plant litter, and decrease in abundance with depth. The zoom-in on a plant root shows how roots release various carbon compounds, called exudates, which feed microbes living in plant rhizospheres. Figure created with BioRender.com by Mara Cloutier.
Because soil microbes are concentrated around plants, including agricultural crops, the activities of soil microbes can have significant effects on soil and crop health. Some microbes are free-living and influence plants through their activities in soil and rhizosphere environments, while other microbes go one step further and form intimate symbiotic associations with plant roots. In some cases, these symbiotic partners reside inside plant tissues themselves. Below, we discuss some of the important ways that both free-living and symbiotic microorganisms influence agricultural systems.
What Agriculturally Relevant Functions do Microbes Perform?
Soil harbors an enormous diversity of microbes, the majority of which remain unknown because we have been unable to capture and study them in laboratory settings. However, we do know that some microbial species promote plant growth by cycling plant nutrients, suppressing plant pests and diseases, and contributing to soil aggregation, porosity, moisture retention, and organic matter accumulation. In contrast, others, known as pathogens, are directly harmful to plant growth by causing plant disease (Table 1).
Table 1: A summary of some activities of soil microbes relevant to crop production.
| Plant growth promoting microbial activities | Harmful microbial activities |
|
|
| General microbial functions in soil | |
|
Plant-Beneficial Microbes
One category of beneficial microbes includes those that increase nutrient availability for crop plants, primarily by transforming plant-unavailable forms of nutrients into a form that plants can use; such microbes are sometimes referred to as biofertilizers. These include nitrogen-fixing bacteria, which transform atmospheric gaseous nitrogen (N2) that plants cannot use into plant-available nitrogen in the form of ammonium (NH4+). One group of nitrogen-fixers called rhizobia form intimate mutualistic relationships with legume plants, living inside specialized root nodules and exchanging the ammonium they produce for carbon inputs from the plant (Fig. 2). Other nitrogen-fixers are free-living in the soil, and still others grow in the rhizosphere or even in the root tissue of non-legume plants such as corn, wheat, and millet. In each case, nitrogen-fixing bacteria increase the nitrogen available for crops to use, thereby boosting plant growth by providing an essential macronutrient.
Another example of a close symbiotic relationship between a microbe and a plant is the partnership between mycorrhizal fungi and plant roots. While there are a few types of mycorrhizal fungi, arbuscular mycorrhizal fungi (AMF) are the type that form relationships with the majority of crop plants. AMF filaments penetrate plant root cells, while also extending through a larger volume of soil than plant roots can reach on their own (Fig. 2). From soil, AMF gather phosphorus and other nutrients, which they transport back to plant roots in exchange for carbon provided by the plant. The AMF-extended root system also substantially improves crop water uptake and drought tolerance.
While AMF are a directly beneficial microbial partner, there are other microbes that can increase the phosphorus available to plants in an indirect manner. Most soil phosphorus is fixed in organic matter, or bound to soil particles as insoluble complexes that are unavailable to plants. In some areas where phosphorus has been added repeatedly to soils, it may exist in high amounts as legacy phosphorus—abundant yet mostly unavailable to plants. Phosphorus-solubilizing microbes produce enzymes or organic acids that release bound phosphorus back into the soil solution, making it generally available for uptake. Similarly, there are many microbes involved in converting nitrogen that is bound in organic matter back into plant-available forms, a process called nitrogen mineralization. This is especially helpful when nitrogen-mineralizing microbes grow in plant root zones, as they can enhance plant nitrogen uptake and minimize the amount of nitrogen lost to leaching.
Another category of beneficial microbes includes those referred to as biocontrol organisms, which suppress plant diseases or attack the insect pests of crops. Some biocontrol microbes act directly against disease-causing microbes (pathogens) by producing antibiotic compounds that kill pathogens. This is the case for biocontrol fungi in the genus Trichoderma, and for many biocontrol bacteria in the Bacillus or Pseudomonas genera. There are also some fungi and bacteria that parasitize or kill insect and nematode pests, such as the bacterium Bacillus thuringiensis, and fungi in the Metarhizium genus. Other biocontrol microbes decrease disease indirectly by outcompeting pathogens for space in the plant rhizosphere, making it difficult for pathogens to initiate root infection. Additionally, some biocontrol microbes are known to activate plant immune systems so that plant tissues have faster and stronger defense responses upon infection, resulting in reduced disease symptoms. This phenomenon is called induced systemic resistance.
Figure 2: Examples of beneficial microbes. The left diagram shows nitrogen-fixing bacteria, housed either in nodules on legume roots or free-living in the soil. The right diagram illustrates arbuscular mycorrhizal fungi (pink) associated with plant roots. Figures credit: Laura Kaminsky.
Besides biofertilizers and biocontrols, some beneficial microbes promote plant growth by influencing plant hormone and metabolic pathways. Such microbes are sometimes called biostimulants, although this term can also refer to nutrient-providing microbes. Plants utilize a number of hormones that regulate their growth patterns, and certain microbes release some of these same hormones into the plant rhizosphere. This can stimulate plant growth by, for instance, increasing the number and length of plant roots. In turn, this supports greater aboveground growth, increases plant water and nutrient uptake, and increases plant drought tolerance. Plants also produce certain molecules when they are under stress (such as during drought events), which constrain their growth. Some microbes are known to degrade these plant stress molecules, allowing plants to maintain higher growth rates even when experiencing stress.
Harmful Microbes
Conversely, there are some microbes whose presence is harmful to crops. The most obvious examples are pathogenic bacteria, fungi, and other microorganisms that cause plant disease. Of these, some microbial pathogens infect plant roots directly from the soil. One example is the bacterium Ralstonia solanacearum, which clogs plant veins and causes wilting symptoms. Other examples are pathogenic fungi in the genera Fusarium and Rhizoctonia, which cause root rot and seedling death. There are also some pathogens which spend part of their life cycle in soil—for instance, by overwintering in the soil as spores—but which infect aboveground parts of crop plants.
In addition to these directly harmful pathogens, some microbes may indirectly limit plant growth by competing with plants for important resources. Microbes have their own nutrient requirements, and can in some instances compete with plants for nitrogen and other soil nutrients. This is especially true following large carbon-rich inputs, such as when straw mulch or high carbon cover crop residues are tilled into the soil. With abundant carbon suddenly available, microbes take up other nutrients at higher rates, which fuels a population growth spurt. Eventually, the nutrients are depleted and the microbial cells die, releasing the nutrients back to the soil. Microbial uptake of nutrients is called immobilization, while microbial release of nutrients is called mineralization. Temporary microbial immobilization of key plant nutrients can limit plant growth, particularly when it happens at critical stages of plant development. However, immobilization can also help conserve and recycle nitrogen and other nutrients in the soil, reducing fertilizer input needs and nutrient runoff into nearby water bodies.
There are also microbes that more permanently deplete soil nutrients. Normally, most microbes use oxygen alongside carbon materials to generate energy for themselves. However, under wet, oxygen-deprived conditions, some microbes switch to using nitrogen compounds in the soil in place of oxygen. This process eventually releases soil nitrogen into the atmosphere as a gas, either elemental N2 or nitrous oxide N2O, in a process called denitrification. This results in a net decrease in soil nitrogen levels, and also contributes significantly to the greenhouse-gas footprint of U.S. agriculture, as nitrous oxide is a powerful greenhouse gas. Soil nitrogen can also be lost if microbes convert it into a water-soluble form such as nitrate—which is then leached from the soil profile—or ammonium which can also be lost to the atmosphere as ammonia gas from alkaline soils.
General Soil Functions Supported by Microbes
Not all microorganisms have direct and obvious effects on plant health and growth. Even so, these neutral microbes can perform general functions that support soil health, which subsequently supports plant health. For instance, many different microbes help process dead plant matter into stable and chemically-diverse organic matter, which in turn results in improved water and nutrient retention and better stabilization against soil erosion. Microbes also support the development of healthy soil structure by helping to form soil aggregates that resist soil compaction, increase soil filtration and water-holding capacity, and promote healthy crop root growth. Fungi are especially important in soil aggregate formation by entangling soil particles in their filaments and gluing soil particles together.
In summary, there are specific microbes known to have either a directly positive or negative effect on plant growth, and there are many more microbes that support more general functions in agricultural soils. Beyond this, there are many soil microorganisms whose activities and functions remain largely unknown because we have so far been unable to grow and study them in laboratory settings.
Is Microbial Diversity a Good Thing?
In the context of soils, the term microbial diversity is often used to refer to two metrics about the community of soil microbes (Fig. 3). The first metric, microbial species richness, refers to the number of unique microbial species in a soil. The second metric, microbial species evenness, refers to whether the community is dominated by only a few abundant species or if there is a more even distribution of species. Determining the relationship between microbial diversity and soil or crop health has long been a goal for scientists studying agricultural soils, but this relationship is not always clear. There are some instances where increased soil microbial diversity has beneficial effects, and other instances where it does not. We highlight some such circumstances below.
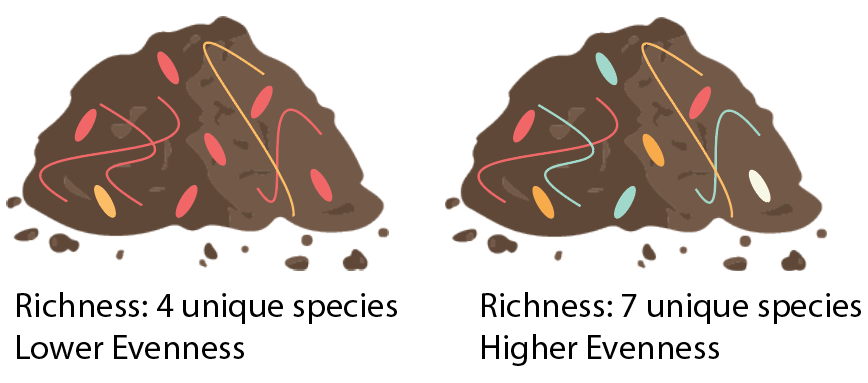
Figure 3: Microbial diversity refers to the number of unique types of microbes in an environment and whether the abundance of each unique species is relatively even or not. In this instance, we would consider the soil sample on the right to contain higher microbial diversity, because it has more unique microbial species and a more even abundance of those species. Image credit: Laura Kaminsky.
When is More Microbial Diversity Better?
In general, soils with higher microbial abundance and diversity have a greater chance of containing individuals that perform a particular function under a range of conditions. As an example, consider the soil nitrogen cycle. Each step in the nitrogen cycle is performed by a different group of microbes, because each step relies on different cellular machinery. Atmospheric nitrogen (N2) is transformed into ammonium (NH4+) by a group of organisms referred to as nitrogen-fixers. Ammonium is also generated by bacteria and fungi that perform decomposition of organic materials, releasing nitrogen that had been bound in more complex molecules. Ammonium-oxidizing microbes then transform NH4+ to NO3- and denitrifying bacteria transform NO3- to N2 and N2O. Complete denitrification (NO3- to N2) often requires multiple different species, as it is uncommon for a particular bacterial species to produce all of the enzymes needed to perform the five-step process. Because many different microbes contribute to each step of this cycle, soils with higher diversity have a greater chance of containing individuals that perform each particular step, and soil nutrient cycling can proceed more efficiently. This is especially relevant for the release of nutrients during decomposition, where the processing of complex organic materials is accelerated by a more diverse group of microbial decomposers, each specializing in a different chemical step in the breakdown of the material.
Another benefit of high microbial diversity is the potential for increased disease suppression. With a greater variety of microbes present, there is a greater chance that there are biocontrol microbes that produce antifungal or antibacterial compounds active against pathogens. Moreover, when a soil or rhizosphere is already inhabited by a diverse microbial community, there tends to be less habitat and fewer resources that an invading pathogen can exploit to establish in the soil. In other words, pathogens can be outcompeted by microbes that already reside in the soil, and may have a reduced ability to infect and cause disease in plants.
Despite the potential benefits of microbial diversity, there may be a saturation point where more diversity does not result in noticeably improved soil function. In this scenario, the addition of a new microbial type is no longer adding a new function, as there is already a resident microbe performing the same function. Even so, this functional redundancy may still increase the chance that soil function will be maintained through an environmental change or disturbance. Even if a disruption of the environment kills off some resident microbes, another microbe performing the same function may survive.
In some cases, high microbial diversity can impact symbiotic plant-microbe relationships (like those formed between plants and rhizobia or AMF) in undesirable ways (Fig. 4). For instance, within AMF and rhizobia, there are individual species that perform better and are considered high-quality partners for particular plants. Performance is based on how many nutrients the microbe gives to the plant in exchange for the carbon that the plant gives to the microbe. If there are both low-quality and high-quality partners present in a soil, there is a chance that plants will form symbioses with low-quality partners as opposed to high-quality partners, although both plant and microbe genetics and behaviors influence partner selection. Therefore, in the case of crops that depend on very specific microbial partners, higher diversity may not be required for optimizing certain functions, provided a high-quality partner is present.
How Can We Assess the Health of Soil Microbial Communities?
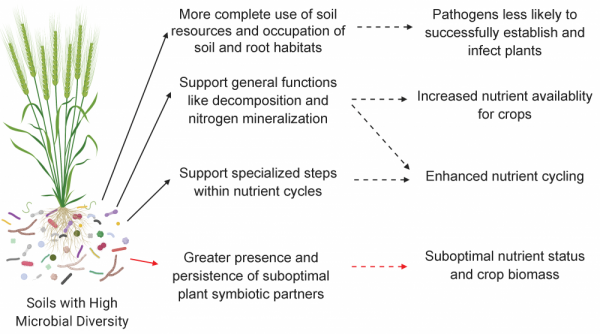
Figure 4: Effects of high microbial diversity in soils. Soils with high microbial abundance and diversity can support pathogen suppression, increase nutrient availability for crops, and enhance nutrient cycling, but may also hinder plants from finding high-quality partners for special symbiotic relationships. Figure credit: Laura Kaminsky.
In a typical agricultural soil test, the results mostly inform us of the chemical and physical properties of a soil sample. Common metrics include soil pH, nutrient levels, soil compaction, and organic matter content. There are clear management suggestions if any of these metrics are subpar, such as fertilization or liming recommendations. Despite the critical role of microorganisms on agricultural production, there is no comparable, standard assessment of soil microbes that is widely available to farmers. While soil microbial communities are very complex and it is difficult to say exactly what constitutes a healthy or unhealthy microbial community, there are some tests that can measure (or estimate) microbial activity.
One of the simplest methods to assess a soil microbial community is to measure soil respiration. Microorganisms release carbon dioxide gas in the process of respiration. The amount of carbon dioxide released from a soil sample in a set amount of time can be measured, which can then be used to estimate the size and activity of the microbial community (Fig. 5). Higher respiration rates are thought to indicate a larger and healthier microbial community, but this metric is imperfect because respiration levels can vary depending on temperature, moisture, and other conditions, and because disturbances such as tillage or the addition of fertilizers can cause a temporary burst of microbial respiration.
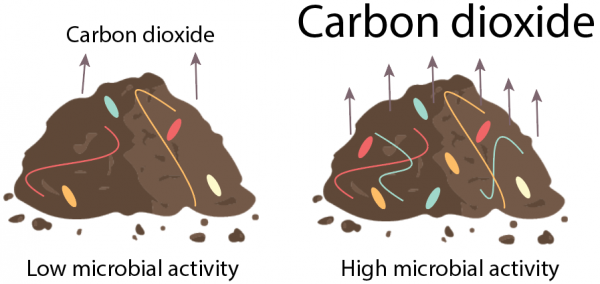
Figure 5: Measuring the amount of carbon dioxide released by soil microbes over a set amount of time is a quick and simple way to determine soil microbial activity. However, this method is not highly precise. Figure credit: Laura Kaminsky.
There are other ways to get a more precise estimate of the size of the microbial community in a soil sample. One method is phospholipid fatty acid analysis (PLFA). The basis behind this test involves cell membranes, which are composed of a double layer of molecules called phospholipids. Phospholipid molecules have a “head” and two “tails.” The tails, which are long chains of carbon molecules, are called fatty acids (Fig. 6). We can extract and measure the amount of fatty acids in a soil sample, which gives us a good estimate of the size of the total microbial population since fatty acids make up such a large component of all cells. There are other methods to estimate microbial biomass, including the commercially available microBIOMETER® soil test (https://microbiometer.com/our-test/), which is based on imaging the presence of microbial cells.
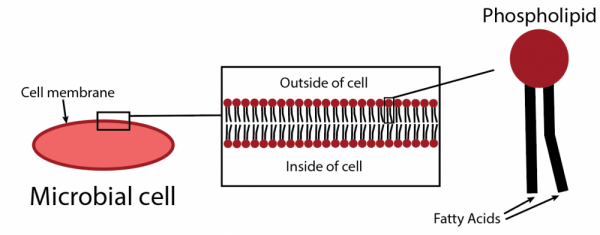
Figure 6: One method for determining microbial biomass is phospholipid fatty acid (PLFA) analysis. By targeting phospholipids, molecules that make up the majority of microbial cell membranes, we can get an estimate of microbial biomass in a soil sample. Figure credit: Laura Kaminsky.
Beyond assessing the abundance of microbes present in a soil sample, it is useful to know which particular microbial species or taxa are present. This can be broadly estimated using PLFA, because the exact chemistry of the fatty acids present in cell membranes varies between different groups of microbes. However, we can inventory microbes in more detail by extracting microbial DNA from soil samples and sequencing certain “barcode” genes. These are genes that all microbes carry, but the exact DNA sequence varies among species. We then match these sequences to a database to determine which sequence belongs to which type of fungi or bacteria (Fig. 7). If we see more of a particular sequence, this indicates that there is more of that particular microbe in the original soil sample. From this catalogue of microbes, we can calculate diversity and learn about the abundance of specific microbes of interest, such as beneficial partners or harmful pathogens.
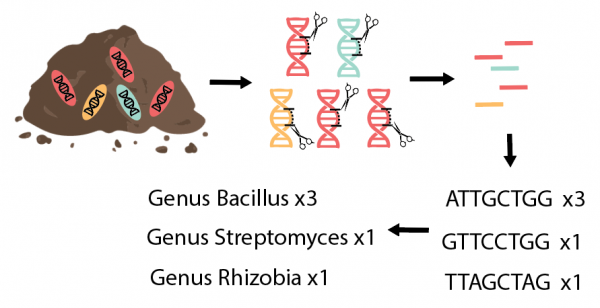
Figure 7: Extraction and sequencing of microbial DNA gives a detailed list of the microbes present in a soil sample. Figure credit: Laura Kaminsky.
While these analyses are informative, we do not know the optimal range for values of soil respiration, microbial biomass, and microbial diversity. Because of this, it can be difficult to provide management recommendations based on these metrics. Even so, these metrics can be useful for tracking how soil management strategies are affecting soil microbial communities across time. Additionally, large multi-group initiatives are working towards understanding how soil management, soil microbial communities, soil health, and plant health are connected. Perhaps the largest ongoing study to address the role of microbial diversity and functions is being conducted by the Soil Health Institute (https://soilhealthinstitute.org/north-american-project/), which studies soils from 125 long-term agricultural research sites across North America. While we continue to study and learn the role of microbes in agricultural soils, tracking how soil management strategies affect soil microbial communities across time will help identify best practices to promote microbes that support soil and plant health.
References and Citations
- Buée, M., W. De Boer, F. Martin, L. Van Overbeek, and E. Jurkevitch. 2009. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant and Soil 321:189—212. (Available online at: https://link.springer.com/article/10.1007/s11104-009-9991-3) (verified 31 Mar 2021).
- Gessner, M. O., C. M. Swan, C. K. Dang, B. G. McKie, R. D. Bardgett, D. H. Wall, and S. Hättenschwiler. 2010. Diversity meets decomposition. Trends in Ecology & Evolution 25:372—380. (Available online at: https://doi.org/10.1016/j.tree.2010.01.010) (verified 31 Mar 2021).
- Hu, J., Z. Wei, V. P. Friman, S. H. Gu., X. F. Wang, N. Eisenhauer, T. J. Yang, J. Ma, Q. R. Shen, and Y. C. Xu. 2016. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. MBio 7(6):e01790-01716. (Available online at: https://doi.org/10.1128/mBio.01790-16) (verified 31 Mar 2021).
- Kallenbach, C. M., S. D. Frey, and A. S. Grandy. 2016. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nature Communications 7:13630. (Available online at: https://www.nature.com/articles/ncomms13630) (verified 31 Mar 2021).
- Kennedy, I. R., A. Choudhury, and M. L. Kecskés. 2004. Non-symbiotic bacterial diazotrophs in crop-farming systems: Can their potential for plant growth promotion be better exploited? Soil Biology and Biochemistry 36:1229—1244. (Available online at: https://doi.org/10.1016/j.soilbio.2004.04.006) (verified 31 Mar 2021).
- Kuzyakov, Y., and X. Xu. 2013. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytologist 198:656—669. (Available online at: https://doi.org/10.1111/nph.12235) (verified 31 Mar 2021).
- Ngumbi, E., and J. Kloepper. 2016. Bacterial-mediated drought tolerance: Current and future prospects. Applied Soil Ecology 105:109—125. (Available online at: https://doi.org/10.1016/j.apsoil.2016.04.009) (verified 31 Mar 2021).
- Raaijmakers, J. M., and M. Mazzola. 2012. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annual Review of Phytopathology 50:403—424. (Available online at: https://doi.org/10.1146/annurev-phyto-081211-172908) (verified 31 Mar 2021).
- Rosenblueth, M., E. Ormeño-Orrillo, A. López-López, M. A. Rogel, B. J. Reyes-Hernández, J. C. Martínez-Romero, P. M. Reddy, and E. Martinex-Romero. 2018. Nitrogen fixation in cereals. Frontiers in Microbiology 9:1794. (Available online at: https://doi.org/10.3389/fmicb.2018.01794) (verified 31 Mar 2021).
- Sharma, S. B., R. Z. Sayyed, M. H. Trivedi, and T. A. Gobi. 2013. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587. (Available online at: https://doi.org/10.1186/2193-1801-2-587) (verified 31 Mar 2021).
- Shoresh, M., G. E. Harman, and F. Mastouri. 2010. Induced systemic resistance and plant responses to fungal biocontrol agents. Annual Review of Phytopathology 48:21—43. (Available online at: https://www.annualreviews.org/doi/10.1146/annurev-phyto-073009-114450) (verified 31 Mar 2021).



