eOrganic author:
Kristen Loria, School of Integrative Plant Science, Cornell University
Introduction
This guide is intended for organic dry bean growers and seed producers to outline practical management options for minimizing risk from important seedborne pathogens. Approximately 50% of all major dry bean pathogens can be seedborne and seed transmitted, making identification and management of seedborne pathogens a substantial issue for dry bean production (Ellis and Gálvez, 1980). While some of these diseases can be managed to some degree in conventional systems using chemical seed treatments and/or applications of pesticides, alternative approaches are critical for successful organic dry bean production.
There are several seedborne bacterial, fungal, and viral pathogens of significance in the United States. Bacterial and fungal pathogens cause the most significant losses in areas of the United States with greater rainfall and higher humidity such as the Midwest, Northeast, and Southeast, because these pathogens favor wet conditions. Some viruses tend to pose a greater threat in arid western regions of the United States where the insect vectors are prevalent. Much of the bean seed supply is produced in arid Western states to reduce the risk of severe bacterial and fungal infections and subsequent seed contamination. While these pathogens do not tend to cause much yield loss in arid regions, they are closely monitored in seed production, as even small amounts of seed contamination can cause substantial disease outbreak when the infected seed is subsequently grown in a humid environment.
Scouting for pathogens, use of relevant cultural practices, and selection of resistant varieties can be effective at mitigating infection of seed crops and crop losses to these pathogens of bean. This guide provides information on identifying some of the more prevalent pathogens in the field, and details practices for preventing initial infection or limiting losses to these pathogens; these practices include crop rotation, phytosanitary measures, use of certified seed, and organic-approved seed treatments. Commercially-available cultivars offering genetic resistance to some seedborne pathogens are also discussed. In general, growing resistant cultivars and planting clean seed are the most effective management tools for preventing infection of bean seed crops.
Major Seedborne Pathogens of Common Bean
In the United States, two major seedborne bacterial pathogens are Xanthomonas axonopodis pv. phaseoli (cause of common bacterial blight) and Pseudomonas syringae pv. phaseolicola (cause of halo blight). Colletotrichum lindemuthianum (cause of anthracnose) is the predominant seedborne fungal pathogen in many areas of the United States, and bean common mosaic virus (BCMV) is one of the most problematic seedborne viruses. There are additional seedborne pathogens of bean that have quarantine and/or other regulatory status in many bean seed production states because of the significance of seedborne inoculum of these pathogens to outbreaks in regions where the seed might be planted. This publication focuses on common bacterial blight, halo blight, anthracnose, and BCMV in organic bean seed production.
All four pathogens can infect all seed tissues, including the embryo. Despite this, “successful seed infection is the exception and not the rule” (Agarwal and Sinclair, 1997), and plants can be infected without seeds becoming infected. However, inoculum is also readily transmitted from a diseased plant to the surface of the seed, such as during harvest of a seed crop (C. Smart, Cornell University, personal communication).
In general, if infection of a bean plant occurs before flowering and conditions remain conducive for the disease, or developing pods show severe symptoms, chances of the seed becoming infected are much greater than on plants with mild symptoms. Severity of mother plant infection is highly correlated with seed infection (Agarwal and Sinclair, 1997). This is the reason that production of bean seed in semi-arid to arid climates has greatly reduced the prevalence of seedborne bacterial and fungal pathogens of bean. Under drier environmental conditions, even if pathogens are present in a field, infections of bean seed crops typically do not get severe enough to infect seed unless the bean seed crops are grown using overhead irrigation.
Bacterial Blights
Some of the bacterial pathogens affecting beans can cause similar symptoms and a bean crop can have simultaneous infections by more than one pathogen, making diagnosis a challenge. Because fungicide-based seed or field treatments are largely ineffective against bacterial pathogens, chemical control options are limited for both organic and conventional bean growers.
Effective diagnosis and management of bacterial pathogens in more humid regions may become even more important as stronger storms and warmer average global temperatures shift the geographic distributions and prevalence of specific pathogens. This is especially favorable for heat-loving bacterial pathogens such as Xanthomonas spp. (Schaad, 2008), a trend that has been observed anecdotally in the Northeast in recent years (C. Smart, Cornell University, personal communication 2019).
Spread of Bacterial Pathogens
Like most bacterial pathogens, bean bacterial diseases proliferate under humid conditions and tend to be more of a problem in wetter and more humid regions such as the Midwest, Northeast, or southeastern United States. Initial infections most commonly occur due to planting infected seed, though inoculum can originate from infected residues present in the field, nearby infected plantings, or infected volunteer bean or weed host plants.
Severe outbreaks can result from even a few initial infected plants. Bacterial pathogens spread within a field or among nearby fields by splashing or wind-driven rain or soil, or by contaminated equipment moving through a crop. Inoculum can travel up to approximately ½ mile through passive processes of wind and splash dispersal, and further distance if transported by humans (e.g., on seed) or equipment. Most plant-pathogenic bacteria must enter the plant through natural openings (e.g., hydathodes or stomata) or wounds. Thus, foliar damage from cultivation or by piercing or sucking insects may increase susceptibility to infection (Agarwal and Sinclair, 1997).
Halo blight and common bacterial blight are regulated by seed certification agencies in most arid seed-producing regions of the western United States, due to the potential of even very low pathogen levels in seed crops leading to potentially severe outbreaks when the infected seed is planted in more humid climates. The increasing use of overhead irrigation for bean seed production in arid environments has also led to an increased incidence of these bacterial diseases in some western regions of the United States (Wohleb and du Toit, 2011).
Very few bacterial pathogens of bean are transmitted from the mother plant to the developing seed through the plant vascular system; most of these pathogens move directly onto the seed coat from an infected pod.
Common Bacterial Blight (Xanthomonas axonopodis pv. phaseoli)
Common bacterial blight is favored by warm temperatures (>80°F) and often appears in July or August in bean seed production regions. This is in contrast to halo blight that more often proliferates in cool to moderate temperatures (Wohleb and du Toit, 2011). Symptoms of common bacterial blight start as small, water-soaked spots and progress to dark brown or reddish-brown lesions, often along the leaf margins because of infection of leaves via the hydathodes. Lesions can become greater than 1 inch in diameter (Figs. 1-3).

Figure 1. Characteristic common bacterial blight lesion with a narrow yellow border. Photo credit: S. Markell, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported.

Figure 2. Mature common bacterial blight lesions are often located at the leaf margins because of infection through the hydathodes. Photo credit: S. Markell, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported

Fig 3. Common bacterial blight lesions on bean pods. Photo credit: J. Pasche, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported
Field scouting for common bacterial blight is typically most effective from 30-45 days after planting, after the crop canopy starts to close and humidity builds up in the canopy (Ellis and Gálvez, 1980). Infected seed does not always display visible symptoms, so it is important to inspect plants in the field for disease symptoms rather than relying on seed inspection after harvest.
Halo Blight (Pseudomonas syringae pv. phaseolicola)
Halo blight is favored by cooler temperatures (<80°F) than common bacterial blight. Symptoms first appear as small (1/16th inch diameter), water-soaked or greasy spots that are later surrounded by a diffuse, greenish-yellow halo (1/16—1/2 inch diameter). The halo is much paler, wider, and more diffuse than the chlorosis typically associated with common bacterial blight lesions, and is caused by the bacterium producing a toxin called phaeolotoxin. Sometimes the halo does not occur, such as in hot weather or if bean plants are infected by atoxigenic strains of the pathogen. Severe infection can cause yellowing of the plant and necrosis of new growth (Schwartz, 2008).

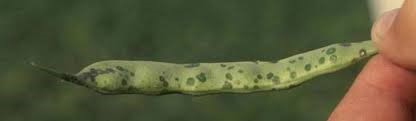
Figure 4. Top image: Water soaked spots on the underside of a bean leaf caused by the halo blight pathogen. Photo credit: R. Harveson, University of Nebraska. Bottom Image: Spots on a pod caused by the halo blight pathogen. Photo credit: S. Markell, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported

Figure 5. Halo blight on leaves: smaller necrotic lesions with diffuse yellow halo. Photo credit: J. Pasche, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported
Fungal Pathogens
Anthracnose (Colletotrichum lindemuthianum)
Symptoms
Anthracnose is caused by a seedborne fungal disease that may result in significant damage, or even complete yield loss. Symptoms on seedlings include small, dark brown and black spots on the cotyledons and rust-colored specks along the hypocotyl and stem, sometimes girdling the seedling. On mature plants, rust-colored lesions form along leaf veins on the underside (abaxial surface) of leaves, with angular brick-red to purple spots on the leaf petiole (Mercure, 1998). Pod symptoms include small, reddish brown to black blemishes, each with a distinctive reddish-brown to black border with a grayish-black interior (Dillard, 1988).


Figure 6. Top - Anthracnose pod lesions. Bottom – White fungal growth on a seed. Photo credit: S. Markell, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported

Fig 7. Anthracnose reddish brown lesions along the leaf veins on the underside of a leaf. Photo credit: S. Markell, North Dakota State University. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported
Spread of Bean Anthracnose
Bean anthracnose is favored by cool to moderate temperatures, high humidity, and extended periods of leaf wetness. The pathogen is transmitted primarily on infected seed, and the duration of survival in soil or on infected plant residues varies, but the pathogen generally is not persistent. Once in a field, fungal spores are disseminated by splashing water, wind-blown rain, and contaminated equipment. While the pathogen survives best on dried bean seed, the fungal spores can survive on bean straw and dried pods.
Seedborne fungi such as C. lindemuthianum most commonly infect the seed coat from an infected pod, and infected seed usually develops visible lesions.
Viruses
Bean Common Mosaic Virus (BCMV)
BCMV is not generally a production problem east of the Rocky Mountains, but can occasionally occur in the eastern United States if seed lots are contaminated and large aphid populations are present in the area. Aphids are the only known insect vector. Symptoms include a mosaic-patterned mottling of the leaves, as well as stunting. If a crop is affected early in the growing season, yield losses of up to 80% can occur.
Virus infection of seed happens during seed development either through systemic infection of the mother plant or pollination of the flowers by BCMV-infected pollen.


Figure 8. Vein banding (left) and mosaic, blistering and distortion of leaves (right) infected with BCMV. Photo credit: R. Harveson, University of Nebraska. Creative Commons Attribution Non-commercial ShareAlike 3.0 Unported. BCMV is largely managed by the use of genetic resistance, which is fairly widespread in modern cultivars.
Management of Seedborne Pathogens in Bean
Especially in organic production systems, the planting of pathogen-free seed and the use of resistant cultivars are the best strategies for limiting damage from seedborne bacterial, fungal, or viral pathogens. Cultural control methods for fungal and bacterial pathogens tend to be similar, but cultural control practices for viruses require different strategies due to the distinct biology of viral pathogens and their modes of transmission.
Cultural Control of Viruses
Viruses are unique in the world of plant pathogens for their tiny size and lack of cellular structures—technically, they are not even considered by some to be alive (Agrios, 2004). They can only survive inside of another organism and rely primarily on insect or animal vectors to be transmitted from plant to plant. Virus-vector relationships tend to be very specific. For BCMV, the only known vector is aphids, so controlling the spread of BCMV in a field requires control of the aphid vectors. Viruses can also be transmitted by mechanical wounding (such as tractors or other equipment moving through a field, or mechanical injury from windstorms), so care should be made not to damage plants when working in the field.
Cultural Control of Bacterial and Fungal Pathogens
Because bacterial and fungal pathogens of bean are spread primarily by water, all practices that promote good air flow in the canopy and minimize water dispersal throughout the field can help limit disease spread. A three-year crop rotation is effective at preventing infection of a subsequent crop from infected field residues as the pathogen only survives in host tissues. Small grains or corn are recommended for rotating with beans as they are not host plants for these bacterial and fungal pathogens. Incorporating crop residues into the soil by disking or plowing accelerates the breakdown of residues and, therefore, any inoculum on the residues. Other management strategies to reduce spread of bacterial and fungal diseases of bean include:
- Use a wider plant or row spacing, if possible (e.g., approximately 30” rows; 2-3 seeds per foot).
- Avoid driving through the field when the foliage is wet (e.g., from rain or dew).
- Clean equipment and tractors before driving between fields.
- Avoid wounding plants by cultivation or other equipment used in a field.
- If irrigation is used, avoid overhead irrigation or schedule irrigation of the crop for the morning so that plants dry out before the cooler evening temperatures.
- Plant the rows in the direction of the predominant wind to take advantage of winds to dry out the canopy between rains or irrigation.
Seed Treatments and Chemical Controls
Hot water treatment has been effective at reducing or eliminating some seedborne pathogens in some smaller-seeded crops such as Brassicas and spinach, but is ineffective in large-seeded crops such as bean because the high temperatures required to kill certain plant pathogens can damage the embryo. However, there is evidence that aerated steam treatment can effectively reduce bacterial and fungal pathogen infestation of vegetables with larger seeds without negatively impacting seed quality (germination and vigor). Aerated steam treatment of seed has the ability to kill pathogens present on the outside of the seed as well as those inside the seed tissue. Further experimentation with aerated steam treatment of bean seed is ongoing, and results to date show that aerated steam treatment reduced disease severity caused by a number of bacterial and fungal pathogens of bean (Darby et al., 2019; Navaratnarn et al., 1980). Check with your seed company or extension specialist for information on access to aerated steam seed treatment. Currently, steam treatment is available as a mail-in service from High Mowing Organic Seeds in Vermont.
Effective organic chemical options for bean seedborne pathogen control are limited. Copper-based products can be applied every 1–2 weeks preventatively for bacterial or fungal infections, if weather conditions favor these diseases. There is some evidence that microbial seed treatment products, including Serenade® and FZB24®, both Bacillus subtilis-based products, were as effective at managing anthracnose in bean crops as a conventional fungicide (Tinivella et al., 2009).
IMPORTANT: Before using any pest control product in your organic farming system:
- Read the label to be sure that the product is labeled for the crop and pest you intend to control, and make sure it is legal to use in the state, county, or other location where it will be applied.
- Read and understand the safety precautions and application restrictions.
- Make sure that the brand name of the product is listed in your Organic System Plan and approved by your USDA-approved certifier. If you are trying to deal with an unanticipated pest problem, get approval from your certifier before using a product that is not listed in your plan—doing otherwise may put your certification at risk.
Note that OMRI and WSDA lists are good places to identify potentially useful products, but all products that you use must be approved by your certifier. For more information on how to determine whether a pest control product can be used on your farm, see the article, Can I Use This Input On My Organic Farm?
As the majority of pathogen inoculum is often present on the exterior of the seed coat, the pathogen load on seed may be reduced by surface-sterilization of the seed immediately before planting. However, this will not eliminate pathogens that are inside the seed coat or cotyledons (embryo).
Sourcing Clean Seed
Ensure that your seed lot for planting is free from these pathogens by buying certified, pathogen-free seed. Bean certification programs are state-based, and are established in many Western states with significant seed industries, such as Idaho, North Dakota, Washington, Oregon, and Colorado.
If you save your own seed for planting purposes or sell your bean crop for use as a seed lot, scout carefully for pathogens during the growing season. However, especially with bacterial pathogens, plants and seeds not displaying symptoms can still produce infected seed (Cafati and Saettler, 1980). This means that seed testing is the safest option for ensuring that your seed is free from pathogens. In some states such as Idaho and Washington, any bean seed planted must have a negative test result for all regulated pathogens in the state. Some labs offering seed pathogen testing include the California Seed and Plant Lab (Pleasant Grove, CA), Eurofins STA Laboratories (Longmont, CO), and Iowa State University's Seed Science Center (Ames, IA). If your seed tests positive for specific seedborne pathogens, you can experiment with treatments such as the aerated steam treatment described above, but the seed should not be sold to other growers unless it has been re-tested and the results are negative.
Planting Disease-Resistant Cultivars
Bean breeding efforts have made great strides in identifying naturally-occurring sources of resistance to important bean diseases, and breeding cultivars with these resistances. Many of these cultivars perform well in organic systems. Genetic resistance as well as upright growth habit are important characteristics for resistance and avoidance of many diseases. Choose varieties that have an upright growth habit that holds pods off the ground and promotes airflow through the canopy. Many black, navy, and pinto bean cultivars have an upright growth habit, as do all kidney and cranberry cultivars.
Heirloom cultivars can vary widely in their genetic susceptibility to specific diseases and in their growth habits. See heirloom variety trials from University of Vermont and Cornell University for information on performance, plant traits, and disease severity (Darby and Cummings, 2017, 2018; Darby et al., 2016; Loria et al., 2019). A significant problem with the increase in production of heirloom varieties for markets is the lack of adequate access to high-quality seed, as large-scale seed production and seed certification in the western United States is limited primarily to seed production for a relatively small number of varieties within modern market classes. Planting beans not intended for use as seed runs the risk of introducing pathogens that can severely impact yields and infect future planting seed lots. Consider contacting your seed supplier(s) to inquire about scouting for these diseases and/or testing the seed for seedborne pathogens that have been conducted on the seed lots.
Commercially Available Varieties with Pathogen Resistance
Resistance to BCMV is widespread in modern bean cultivars. Introduction of resistance to common bacterial blight and anthracnose in commercial cultivars has proven more challenging, but moderate to high resistance is becoming available. Most navy, black, and pinto bean cultivars (from the Middle American domestication center) are resistant to halo blight, while many kidney or cranberry cultivars (from the Andean domestication center) are susceptible.
Table 1. Commercially Available Bean Cultivars with Resistance to Different Diseases. S = susceptible; R = resistant (complete or partial resistance) to that specific pathogen. (Ghising & Horsley, 2016; Kelly et al., 2019; Kelly et al., 2018; E. Wright and J Kelly, personal communication, 2020).
| Variety | Market Class | Anthracnose | Common Bacterial Blight | Halo Blight | BCMV |
|---|---|---|---|---|---|
| Rio Rojo (NDSU) | Small Red | S | High R | Moderate R | R |
| Cayenne | Small Red | S | Moderate R | Moderate R | R |
| OAC Candycane | Cranberry | R (race 73) | S | Unknown | R |
| Red Cedar | Dark Red Kidney | R (race 7, 73) | Moderate-high R | Likely resistant | R |
| Red Hawk | Dark Red Kidney | R (race 7, 73) | S | Resistant | R |
| Coho | Light Red Kidney | R (race 73) | Moderate-high R | Likely resistant | R |
| Zorro | Black | R (race 7) | Moderate R | Likely R | R |
| Zenith | Black | R (race 7, 73) | Moderate R | Likely R | R |
| Powderhorn | Great Northern | S | Moderate R | Moderate R | R |
| OAC Rex | Navy | S | High R | Moderate R | R |
| Bolt | Navy | R (race 73) | S | Moderate R | R |
| Orca | Specialty | Unknown | Unknown | Moderate R | R |
Seed Sources for Organic and Specialty Seed
It can be difficult to find specialty or organic bean seed in quantities sufficient for commercial planting (i.e., in fields of > ¼ acre). Variety options for certified pathogen-free seed are currently limited to major market classes. The following are companies that sell seed in larger quantities, some offering major market class varieties and some focused on heirloom and specialty types. It is important to inquire about seedborne pathogen testing procedures to ensure the seed purchased is pathogen-free, particularly if the seed is to be planted in regions with state quarantines or other phytosanitary regulations around seedborne pathogens of bean.
- Adaptive Seeds, Sweet Home, OR
- AP Whaley, Mount Horeb, WI
- Central Bean, Quincy, WA
- Fedco Seeds, Clinton, ME
- Gen-Tec, Twin Falls, ID
- High Mowing Organic Seeds, Wolcott, VT
- Hudson Valley Seed Company, Accord, NY
- Seed Savers Exchange, Decorah, IA
- Uprising Organics, Bellingham, WA
- Turtle Tree Seeds, Copake, NY
- Vermont Bean Co., Randolph, WI
- Victory Seeds, Molalla, OR
References and Citations
- Agarwal, V. K., and J. B. Sinclair. 1997. Principles of seed pathology. 2nd ed. Lewis Publishers, Boca Raton, FL.
- Agrios, G. 2004. Plant pathology. 5th ed. Academic Press.
- Cafati, C. R., and A. W. Saettler. 1980. Transmission of Xanthomonas phaseoli in seed of resistant and susceptible Phaseolus genotypes. Phytopathology 70:638—640. Available online at: https://www.apsnet.org/publications/phytopathology/backissues/Documents/1980Abstracts/Phyto70_638.htm (verified 22 Mar 2021).
- Darby, H., and E. Cummings. 2017. 2016 Heirloom dry bean variety trial [Online]. Northwest Crops and Soils Program, University of Vermont. Available at: https://scholarworks.uvm.edu/nwcsp/121/) (verified 22 Mar 2021).
- Darby, H., &and E. Cummings. 2018. 2017 Heirloom dry bean variety trial [Online]. Northwest Crops and Soils Program, University of Vermont. Available at: https://www.uvm.edu/search_results?q_as=2016%20Heirloom%20dry%20bean%20variety%20trial (verified 22 Mar 2021).
- Darby, H., E. Cummings, L. Calderwood, A. Gupta, J. Post, and S. Ziegler. 2016. 2015 Heirloom dry bean variety trial [Online]. Northwest Crops and Soils Program, University of Vermont. Available at: https://www.uvm.edu/search_results?q_as=2016%20Heirloom%20dry%20bean%20variety%20trial (verified 22 Mar 2021).
- Darby, H., R. Malone, E. Cummings, and H. Emick. 2019 [Online]. The effects of seed steam treatment on dry bean yield and presence of pests & disease. Available at https://www.uvm.edu/sites/default/files/media/2018_Dry_Beans_Steam_Treatment.pdf (verified 22 Mar 2021).
- Dillard, H. 1988. Bean Anthracnose fact sheet [Online]. Fact Sheet 729.40. Vegetable MD Online, Cornell University. Available at http://vegetablemdonline.ppath.cornell.edu/factsheets/Beans_Anthracnose.htm (verified 22 Mar 2021).
- Ellis, M. A., and G. E. Gálvez. 1980. Seed pathology. In G. E. Schwartz, F. Howard F. and E. Gálvez E. (eds.) Bean production problems: Disease, insect, soil and climatic constraints of Phaseolus vulgaris. Centro Internacional de Agricultura Tropical (CIAT).
- Ghising, K. 2016. Screening of the USDA core collection of common bean for reaction to Halo blight and identification of genomic regions associated with resistance. Doctoral Dissertation, North Dakota State University.
- Kelly, J. D., E. M. Wright, G. V. Varner, M. I. Chilvers, and C. L. Sprague. 2019. “Coho”: A new light red kidney bean for Michigan. East Lansing.
- Kelly, J. D., G. V. Varner, M. I. Chilvers, K. A. Cichy, and E. M. Wright. 2018. Registration of 'Red Cedar' dark red kidney bean. Journal of Plant Registrations 12:199—202. Available online at: https://doi.org/10.3198/jpr2017.05.0034crc (verified 22 Mar 2021).
- Loria, K., M. Mazourek, and G. Inzinna. 2019. 2019 Dry bean variety trial [Online]. Cornell University, Ithaca, NY. Available at: https://varietytrials.eorganic.info/sites/eorg-variety7/files/Dry%20Bean%20Variety%20Trial%20Report%202019%20%28NY%29.pdf (verified 22 Mar 2021).
- Mercure, P. 1998. Anthracnose of bean [Online]. Integrated Pest Management Program, University of Connecticut. Available at http://ipm.uconn.edu/documents/raw2/Anthracnose of Bean/Anthracnose of Bean.php?aid=116 (verified 23 Mar 2021).
- Navaratnarn, S. J., D. Shuttleworth, and D. Wallace. 1980. The effect of aerated steam on six seed-borne pathogens. Australian Journal of Experimental Agriculture and Animal Husbandry 20:97—101. (Abstract available onlinle at: https://doi.org/10.1071/EA9800097 (verified 23 Mar 2021).
- Schaad, N. W. 2008. Emerging plant pathogenic bacteria and global warming. p. 369—379. In Fatmi, M., Collmer, A., Iacobellis, N.S., Mansfield, J., Murillo, J., Schaad, N.W., Ullrich, M. (eds.) Pseudomonas syringae Pathovars and Related Pathogens—Identification, Epidemiology and Genomics. Springer Netherlands. (Available for purchase at: https://link.springer.com/chapter/10.1007/978-1-4020-6901-7_38 (verified 23 Mar 2021).
- Schwartz, H. 2008. Bacterial diseases of beans [Online]. Bugwood Wiki. Available at https://wiki.bugwood.org/Bacterial_diseases_of_beans (verified 23 Mar 2021).
- Tinivella, F., L. M. Hirata, M. A. Celan, S.A.I. Wright, T. Amein, A. Schmitt, E. Koch, J. M. van der Wolf, S.P.C. Groot, D. Stephan, A. Garibaldi, and M. L. Gullino. 2009. Control of seed-borne pathogens on legumes by microbial and other alternative seed treatments. European Journal of Plant Pathology 123:139—151. (Available online at: https://www.researchgate.net/publication/227296499_Control_of_seed-borne_pathogens_on_legumes_by_microbial_and_other_alternative_seed_treatments (verified 23 Mar 2021).
- Wohleb, C., and L. du Toit. 2011. Common bacterial blight and halo blight: Two bacterial diseases of phytosanitary significance for bean crops in Washington State. Fact Sheet FS038E, Washington State University Extension. (Available online at: http://mtvernon.wsu.edu/path_team/FS038E-CommonBacterialBlightAndHaloBlight.pdf (verified 23 Mar 2021).
This work was made possible by a Northeast SARE Graduate Research Grant




