eOrganic authors:
Ben Samuelson, University of Nebraska-Lincoln
Samuel Wortman, University of Nebraska-Lincoln
Rhae Drijber, University of Nebraska-Lincoln
This article reviews popular ideas surrounding compost extract, defines terms and relevance to organic production, and presents the conclusions of a project that explored biological and chemical characteristics of different compost extracts and tested their efficacy. We highlight the variability among compost extracts and the challenges related to testing the usefulness of any particular compost extract.
For this article, compost extract refers to a preparation of compost and water with neither additives nor a fermentation period. Related preparations have been used widely in many contexts and for many intended purposes. Biodynamics, Korean natural farming, and other traditional approaches include preparations similar to compost extract, and many commercially available microbial biostimulants contain organisms that may also be present in compost extract.
Preparation and Use of Compost Extract
A common method used to make compost extract is to knead a nylon mesh bag of compost in a basin of water. Any apparatus that agitates while filtering can be used, as long as the result is a liquid that contains soluble and particulate material derived from the compost.
Any ratio of compost to water may be used, with the resulting compost extract containing a proportionate amount of particulate. For application by drip irrigation, the particulate should first be filtered or allowed to settle out. Presence and size of particulate is less relevant when using other methods of application, such as a hydroseeder. Common application methods include soil drench, foliar spray, seed treatment, or as a crop residue treatment.
Compost Extract in Organic Systems
Compost extract is proposed as a biological inoculum that can suppress disease, increase soil health, increase soil microbial abundance and/or diversity, promote plant growth, accelerate residue decomposition, increase nutrient cycling, and even suppress weeds (Lowenfels, 2010; Zinati, 2018).
In a review of primary research, Litterick et al. (2004) suggested that compost extract may be suited to organic production systems due to limitations on the use of synthetic pesticides and fungicides. However, from a compliance perspective, a clear definition of compost extract has not been put forward by the USDA National Organic Program (USDA-NOP), and there is no explicitly allowed nor prohibited way to use compost extract. However, non-synthetic inputs are allowed (per NOP regulation 205.105), so as long as extracts are derived from allowed organic feedstocks, then the extract should also be allowed (since the only additional ingredient is water). The more important regulatory question is whether a particular compost extract can be applied to a crop grown for human consumption. Any extract derived from compost containing animal manure or other animal-based materials that does not meet the NOP definition and requirements for compost (minimum time and temperature) would be restricted with a 90 day post-harvest interval when applied to crops for human consumption. Thus, it is important that any compost extract and the procedure for its preparation be listed in every farm's Organic System Plan, and that growers check with their certifier before adopting compost extract for any particular use or crop.
IMPORTANT: Before using any pest control product in your organic farming system:
- Read the label to be sure that the product is labeled for the crop and pest you intend to control, and make sure it is legal to use in the state, county, or other location where it will be applied.
- Read and understand the safety precautions and application restrictions.
- Make sure that the brand name product is listed in your Organic System Plan and approved by your USDA-approved certifier. If you are trying to deal with an unanticipated pest problem, get approval from your certifier before using a product that is not listed in your plan—doing otherwise may put your certification at risk.
Note that, although OMRI and WSDA lists are good places to identify potentially useful products, all products that you use must be approved by your certifier. For more information on how to determine whether a pest-control product can be used on your farm, see the related article, Can I Use This Input On My Organic Farm?
Possible Mechanisms of Action
Fertilizer benefits of compost are well understood, but compost extract is often used at such low rates that its nutrient addition might be considered negligible. Instead, its effects tend to be attributed to its function as a microbial inoculant. For example, some compost microorganisms have been shown to be antagonistic to plant pathogens (Neher et al., 2017; McKellar & Nelson, 2003). Moreover, compost extracts tend to lose their disease-suppressive properties when sterilized, suggesting that microbes in compost are critical to the use of extracts for disease suppression (Neher et al., 2017; El-Masry et al., 2002). When this research is combined with many personal anecdotes on YouTube and gardening blogs, it is not surprising that compost extract is claimed in the popular press to be greatly beneficial (Lowenfels & Lewis, 2010). However, compost extract is subject to unpredictable variability between production systems, the compost extracts themselves, and the specifics of how they are applied.
How can a producer have confidence that their unique compost extract will be effective for a specific use? This article describes our original research investigating this question. Our broadest take-home message is that while compost extracts sometimes have notable positive effects, how to predict whether a particular compost extract will work for a particular purpose is still unknown. Compost extracts vary widely in chemical and biological properties (no two extracts are identical), which makes it difficult to predict or explain effects.
Our Original Research on Compost Extract
The purpose of our research study was to describe the range of testable biological and chemical differences among ten compost extracts, and to determine whether any of these attributes predicted seedling performance, disease suppression, or plant growth. We also aimed to cross-validate testing methods: Does one test for microbial content agree with a different kind of test? All ten compost extracts were tested for phytotoxicity to lettuce seedlings, and seven compost extracts were tested for Pythium damping-off suppression when used as a cucumber seed drench. Additionally, three compost extracts were selected for a greenhouse experiment to evaluate their effect on crop growth when applied to different crop residues prior to soil incorporation.
Composts Evaluated
Composts were selected for feedstock diversity and production process (Table 1). Additional treatments of municipal sewage sludge (Biosolids) and an immature feedlot compost (ACN Inwood) were included because of their widespread use on many crop acres, despite their prohibition or restriction under organic rules. Compost extracts were prepared in water using an equal dry weight of compost. Moisture content of each compost was determined, then fresh compost containing 100 g dry matter was added to a 400 µm nylon mesh bag and kneaded in a total adjusted volume of 1000 mL of water.
Table 1: Name, short description of feedstock and production process, availability, and price of composts used for preparing compost extracts
| Name | Description | Availability | Approximate $/lb |
|---|---|---|---|
| Biosolids | Wastewater sludge from biodigester | bulk | $0.00 |
| NPL Mushroom | Spent mushroom substrate | bagged | $0.11 |
| Big Red Worms | Food/yard waste vermicompost | bagged | $1.00 |
| EKO Compost | Poultry manure/wood windrow | bagged | $0.32 |
| David Johnson | Static yard waste vermicompost | backyard | n/a |
| Wiggle Worm | Vermicompost from organic grain | bagged | $1.15 |
| Soil Dynamics | Municipal windrow | bulk | $0.02 |
| ACN Inwood | Cow manure/corn stover windrow | bulk | $0.01 |
| Home Worms | Household vermicompost | backyard | n/a |
| Mountain Magic | Cow manure/forest windrow | bagged | $0.11 |
Chemistry of Compost Extracts
Nutrient analysis and other chemical properties were analyzed by Ward Labs (Kearney, NE). Table 2 shows the high variability of nutrient concentrations present in diverse compost extracts. Composts are known to vary greatly in chemical composition, and our research confirmed that the extracts of those composts are similarly variable. The chemistry of the solid compost closely matched that of its corresponding compost extract (data not shown). Two composts, indicated in Tables 2 and 3, were determined to be immature due to their high ammonium content and rapid CO2 respiration (data not shown).
Table 2: Chemical parameters of compost extracts. Maximum and minimum values among extracts of mature composts are in red and blue, respectively. “Range ÷ High” represents the ratio of the range among observed values to the highest observed value within extracts of mature composts.
| Compost Extract | Total N (ppm) | Total P (ppm) | Total K (ppm) | Sulfur (ppm) | Calcium (ppm) | Magnesium (ppm) | pH | C:N | |
|---|---|---|---|---|---|---|---|---|---|
| Immature Composts | ACN Inwood | 1129 | 1684 | 1895 | 566 | 2195 | 873 | 8 | 9 |
| Biosolids | 2689 | 2325 | 119 | 993 | 2021 | 221 | 6.6 | 5.9 | |
| Mature Composts | NPL Mushroom | 1306 | 298 | 281 | 306 | 2463 | 566 | 7.4 | 14 |
| Big Red Worms | 822 | 450 | 959 | 133 | 1473 | 294 | 7.9 | 14 | |
| EKO | 594 | 1271 | 1076 | 204 | 2019 | 245 | 8.8 | 11 | |
| David Johnson | 891 | 275 | 65 | 224 | 2343 | 188 | 7.9 | 15 | |
| Wiggle Worm | 1109 | 78 | 73 | 296 | 1322 | 252 | 6.6 | 14 | |
| Soil Dynamics | 655 | 238 | 600 | 67 | 730 | 197 | 8 | 8.2 | |
| Home Worms | 1434 | 595 | 1601 | 210 | 1877 | 357 | 7.4 | 12 | |
| Mountain Magic | 321 | 157 | 419 | 106 | 1894 | 382 | 8.2 | 28 | |
| Range ÷ High among mature composts' extracts | 0.78 | 0.94 | 0.95 | 0.78 | 0.7 | 0.66 | 0.3 | 0.7 | |
Biological Tests
Any expectation of an inoculant effect of a compost extract depends on the presence of living organisms. We therefore attempted to characterize the microbial component in our compost extracts.
Several methods were used to evaluate agreement among methods for measuring microbial content of our compost extracts. Total microbial biomass was estimated using Fatty Acid Methyl Ester extraction (FAME)(Grigera et al., 2006; Grigera et al., 2007); the Soil Microbiometer® system (Prolific Earth Sciences, Montgomery, NY); and direct microscopic observation according to the method of Ingham and Klein (1984).
Our evaluation indicated that compost extracts varied widely in microbial content (Table 3). Moreover, the method used to estimate a microbial parameter had a strong influence on the result. For example, Wiggle Worm compost had the least total FAMEs (biochemical marker for microbial abundance), but the greatest microbial biomass estimated by the Soil Microbiometer® system.
Table 3: Ranges of biological parameters across 8 finished composts. “Range ÷ High” represents the ratio of the range across observed values to the highest observed value within extracts of mature composts.

Overall, there is rather poor agreement between methods for measuring microbial biomass in compost extract. A positive correlation between methods would indicate that if one measurement is high, there is a good chance the other will also be high. There was a significant correlation between total microbial biomass by microscopic count and FAME (r = 0.82, p = 0.023; Figure 1). Other correlations within Table 3, including fungal:bacterial (F:B) biomass ratio between FAME and microscopy were not significant. Microscopy and FAME approaches had significant positive correlations for bacterial biomass, but not for fungal biomass (data not shown). The Soil Microbiometer®, a system designed to measure soil microbial biomass, did not correlate well with FAME or microscopy estimates of compost extract microbial abundance. This illustrates that different methods for testing biological content of compost extract may result in very different perspectives on the character of the extract.
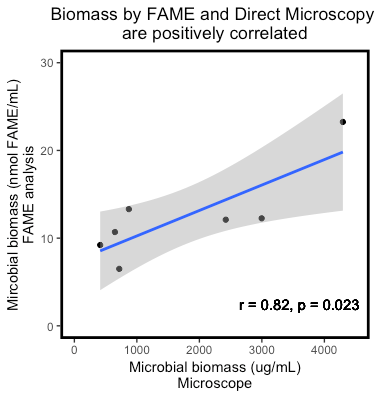
Figure 1: Scatter plot with trendline in blue showing a significant positive correlation between microbial biomass as estimated by FAME analysis and direct microscopic count. The gray area indicates a 95% confidence interval for the slope of the trendline. Analysis by Pearson's product-moment correlation.
Lettuce Seed Germination
Using a quick and simple test to determine whether compost extract hinders lettuce seed germination, we prepared four replicates of ten lettuce seeds in Petri dishes on filter paper moistened with each compost extract. The number of seedlings germinated was recorded daily for three days. Among the extracts, only municipal biosolids hindered lettuce seed germination, and ACN Inwood markedly slowed root elongation. We suspected that ammonium toxicity caused this result. These two composts had the greatest proportion of their total-N present as ammonium, as well as relatively high CO2 production when dried and rewetted—both are indicators of immaturity. They were excluded from further trials.
Pythium Damping-Off Suppression
Several studies have described suppression of Pythium damping-off by certain composts, and lack of suppression by others, mainly attributable to bacterial groups (McKellar & Nelson, 2003; Scheuerell & Mahaffee, 2005). We tested Pythium suppression by our compost extracts made from mature composts. Cucumber seeds were soaked in each compost extract for one minute. The seeds were then sown in a seeding mix heavily infested with Pythium and grown at high humidity to favor pathogen virulence. Significantly different levels of Pythium suppression were found (Fig. 2). Although the fungal:bacterial (F:B) biomass ratio has been proposed as a general predictor of disease-suppressing power of compost extracts, we found that the compost extracts with the greatest F:B biomass ratio resulted in the least suppression of Pythium (Table 3).
Figure 2: Percent healthy seedlings resulting from cucumber seeds soaked for one minute in various compost extracts and water control, grown to one true-leaf stage in media heavily infested with Pythium sp. Columns that do not share a lower-case letter are different (p < 0.05).
These results might suggest that compost extracts with abundant fungal hyphae are unlikely to suppress Pythium damping-off. Another conclusion could be that some compost extracts may suppress damping-off in seedlings, which would contradict the common practice of managing seedlings as aseptically as possible by using pasteurized media and avoiding compost in controlled environments.
Treating Crop Residues with Compost Extract
This experiment tested whether different compost extracts applied to crop residues could impact lettuce growth. Aside from disease antagonism, another common expectation of compost extract is that it can alter soil microbial communities to improve soil health and crop growth. However, previous research suggests relatively small doses of microbes applied as inoculants have limited potential to alter long-term soil microbial community structure; rather community structure is more often influenced by microbial habitat, namely soil texture and structure, moisture, and temperature (Pankhurst et al., 2002; Wortmann et al., 2008). Important exceptions are cases when inoculations are well-targeted in both application and function, as in Rhizobium spp. seed treatments for legumes.
One such targeted application and function of compost extract biology may be in the management of agricultural residues. Accumulation of agricultural residues in soil can be problematic, especially if those residues have a high C:N with the potential for nitrogen immobilization. Immobilization occurs in high C:N soil environments where microbial communities are efficient at scavenging for nitrogen and store or immobilize it within their cells (Mooshammer et al., 2014). Cover crops and biodegradable mulch residues rich in carbon, such as rye or cellulose-based mulch, can lead to N immobilization and limit N availability to following cash crops (Parr and Papendick, 1978). Protozoa and nematodes are known to accelerate mineralization of nutrients in residues by consuming decomposer bacteria and fungi and excreting plant-available nutrients (Bonkowski & Clarholm, 2012; Santos et al., 1981). Our objective was to use compost extracts to speed the residue decomposition process and mitigate the worst effects of N immobilization on subsequent crops. We hypothesized that compost extract applied directly to residues prior to soil incorporation would improve growth of subsequent crops planted into various agricultural residues (in this case, lettuce).
To test this, we simulated a management approach in which compost extract would be sprayed on a field of biodegradable mulch fabric, oat straw, or green manure cover crop (alfalfa) just before incorporation by tillage. This could be accomplished by a tractor with sprayers on the front and a disc implement pulled behind. This study was conducted in the greenhouse and residues were sprayed by hand before mixing into soil.
We simulated this in a greenhouse experiment by spraying residues before mixing into soil. The compost extract factor contained five treatments selected to represent a range of expected biological characteristics as estimated by microscopy methods: Home Worms, Soil Dynamics, EKO compost, urea as a positive control for N, and a water control. Compost extracts were selected to represent a range of fungal biomass and microbial predator populations: Home Worms, Soil Dynamics, and EKO compost. The residue factor contained five treatments: alfalfa, oat straw, biodegradable mulch composed of wood particles and polylactic acid (PLA), polypropylene geotextile as a control, and a no-residue control. The design was a 5x5 full factorial randomized complete block design (RCBD) with six replicates (Rzewnicki, 1992). All extracts and the urea control were applied at rates to supply 3 lb/ac (3.36 kg/ha) of N. Residues were applied at realistic field rates for green manure (5 ton/ac), oat residue (2 ton/ac), and mulch fabric (1.67 ton/ac), incorporated to 4-inch depth in 4x4-inch pots. Lettuce was sown two weeks after incorporation, and above-ground dry weight was measured 42 days after planting.
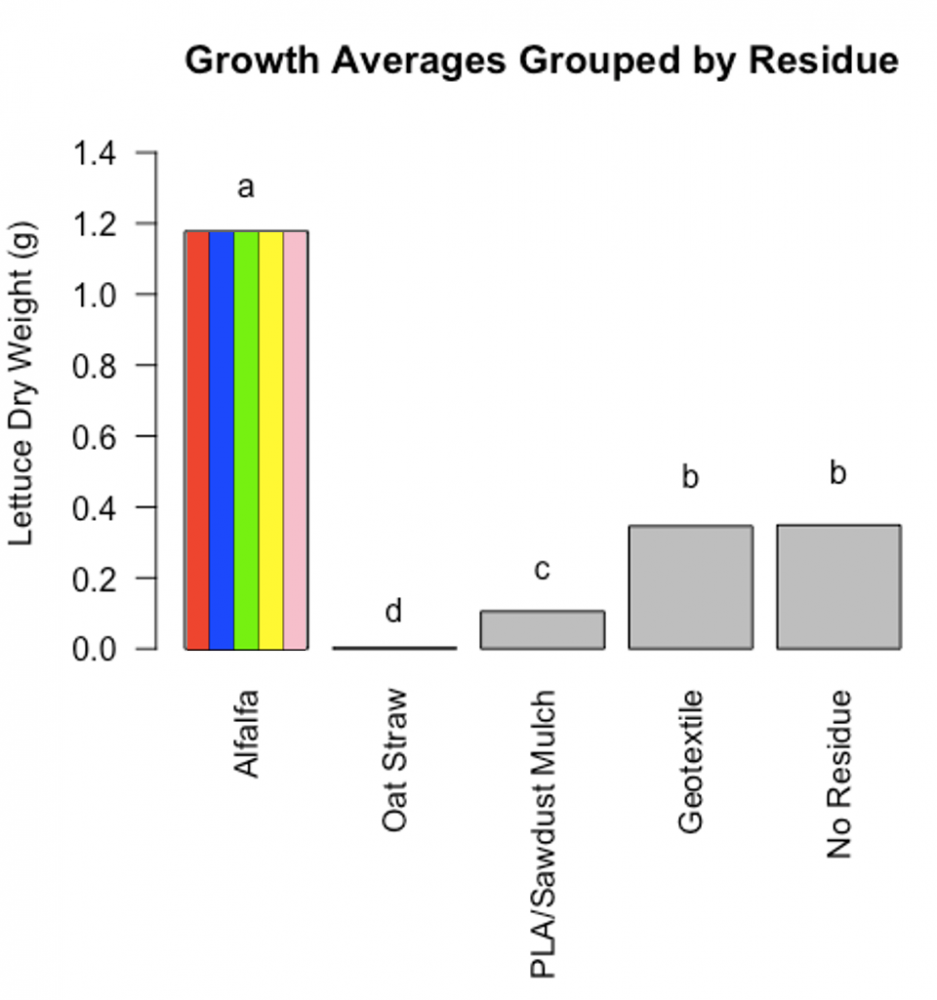
Figure 3: Significant main effects due to residue are shown. Columns represent weight of lettuce above-ground dry biomass of each residue treatment, averaged across compost extract. Columns sharing the same lower-case letter are not significantly different (P<0.05). The alfalfa column is colored to illustrate that it represents the average of the five extract treatments within the alfalfa residue which are shown separately in Figure 4.
We expected that abundant predators in compost extract (e.g., Home Worms) would overcome the expected nutrient immobilization due to incorporation of high C:N residues (oat straw and PLA/wood mulch). However, all three of our compost extract inoculations failed to overcome nutrient limitations resulting from these high C:N residues with overall poor lettuce growth
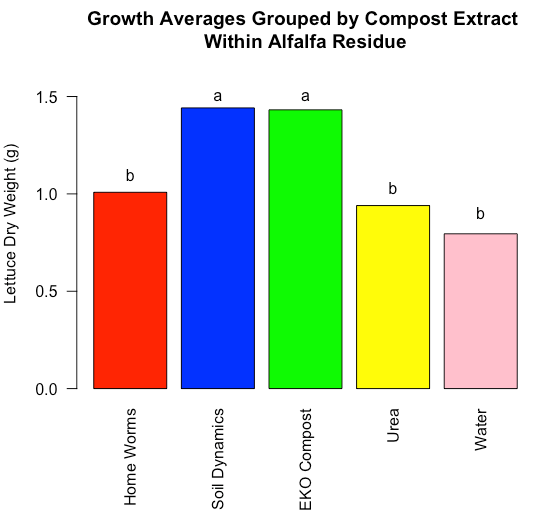
Figure 4: Dry weight of lettuce grown in soil with incorporated alfalfa residue treated with compost extracts. Columns sharing the same lower-case letter are not significantly different (P<0.05).
Alfalfa residue in soil increased lettuce growth overall. This is largely due to N contributions from the alfalfa. However, this effect was also modulated by choice of compost extract (Fig. 4). Our expectation of increased growth due to more fungi and predators (protozoa and nematodes) as measured in the Home Worms compost extract was rejected. Rather, the two compost extracts that were estimated to contain less desirable biological traits increased growth by 53% over the urea and water controls.
Summary
We have shown that compost extract is highly variable in chemical and microbiological properties, and our results are similar to others' findings that in certain cases compost extract can suppress Pythium spp., and cause changes in plant growth unrelated to N input. However, we are still unable to suggest tests or criteria for predicting compost extract usefulness.
Deciphering microbial ecological systems is difficult, and our understanding of soil and compost microbes is limited by the methods used to measure them. We showed that available methods for estimating microbial parameters of compost extract do not agree closely with one another. We failed to find, in this work or in the literature, highly dependable predictors of compost extract efficacy for any particular use.
Compost extract is potentially allowable in organic systems if derived from allowable compost feedstock, but must be listed in every farm's organic system plan. Producers should check with their certifier before using it. Some benefits are possible, namely disease suppression and enhanced plant growth, and it is rare that compost extract made from mature organic-allowable compost will result in detrimental agronomic effects. However, given the unpredictability of benefits, we do not advise use of compost extracts on a commercial scale unless benefits of a specific extract are first documented through site-specific on-farm research.
Acknowledgements
This project is based on research that was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Act (accession 1014303) through the USDA National Institute of Food and Agriculture (NIFA), the North Central Regional Sustainable Agriculture Research and Education Graduate Student Grant Program (GNC17-250), and the USDA NIFA Organic Transitions Program (award 2016-51106-25711). Gratitude to Elizabeth Jeske for her considerable expertise and assistance with FAME analysis.
For more information or for data not included in this article, send correspondence to M. Benjamin Samuelson samuelsonmb@gmail.com
References
- Bonkowski, M., and M. Clarholm. 2012. Stimulation of plant growth through interactions of bacteria and protozoa: Testing the auxiliary microbial loop hypothesis. Acta Protozoologica 51:237—247. (Available online at: https://doi.org/10.4467/16890027AP.12.019.0765 (verified 14 Aug 2019).
- El-Masry, M. H., A. I. Khalil, M. S. Hassouna, and H.A.H. Ibrahim. 2002. In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World Journal of Microbiology and Biotechnology 18:551—558. (Available online at: https://link.springer.com/article/10.1023/A:1016302729218 (verified 14 Aug 2019).
- Grigera, M. S., R. A. Drijber, K. M. Eskridge, and B. J. Wienhold. 2006. Soil microbial biomass relationships with organic matter fractions in a Nebraska corn field mapped using apparent electrical conductivity. Soil Science Society of America Journal 70:1480—1488. (Available online at: https://doi.org/10.2136/sssaj2005.0331 (verified 18 Sep 2019).
- Grigera, M., R. A. Drijber, R. H. Shores-Morrow, and B. J. Wienhold. 2007. Distribution of the arbuscular mycorrhizal biomarker C16:1cis11 among neutral, glyco and phospholipids extracted from soil during the reproductive growth of corn. Soil Biology and Biochemistry 39:1589—1596. (Available online at: https://doi.org/10.1016/j.soilbio.2007.01.009 (verified 14 Aug 2019).
- Ingham, E. R., and D. A. Klein. 1984. Soil fungi: Relationships between hyphal activity and staining with fluorescein diacetate. Soil Biology and Biochemistry 16:273—278. (Available online at: https://doi.org/10.1016/0038-0717(84)90014-2 (verified 14 Aug 2019).
- Litterick, A. M., L. Harrier, P. Wallace, C. A. Watson, and M. Wood. 2004. The role of uncomposted materials, composts, manures, and compost extracts in reducing pest and disease incidence and severity in sustainable temperate agricultural and horticultural crop production—A review. Critical Reviews in Plant Sciences 23:453—479. (Available online at: https://doi.org/10.1080/07352680490886815 (verified 14 Aug 2019).
- Lowenfels, J., and W. Lewis. 2010. Teaming with microbes: A gardener's guide to the soil food web. Timber Press, Portland, OR.
- McKellar, M. E., and E. B. Nelson. 2003. Compost-induced suppression of Pythium damping-off is mediated by fatty-acid-metabolizing seed-colonizing microbial communities. Applied and Environmental Microbiology 69:452—460. (Available online at: https://doi.org/10.1128/AEM.69.1.452-460.2003 (verified 14 Aug 2019).
- Mooshammer, M., W. Wanek, I. Hämmerle, et al. 2014. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nature Communications 5:3694. (Available online at: https://www.nature.com/articles/ncomms4694 (verified 18 Sep 2019).
- Neher, D. A., L. Fang, and T. R. Weicht. 2017. Ecoenzymes as indicators of compost to suppress Rhizoctonia Solani. Compost Science & Utilization 25:251—261. (Available online at: https://doi.org/10.1080/1065657X.2017.1300548 (verified 14 Aug 2019).
- Pankhurst, C., C. Kirkby, B. Hawke, and B. Harch. 2002. Impact of a change in tillage and crop residue management practice on soil chemical and microbiological properties in a cereal-producing red duplex soil in NSW, Australia. Biology and Fertility of Soils 35: 189—196. (Available online at: https://doi.org/10.1007/s00374-002-0459-3 (verified 14 Aug 2019).
- Pant, A. P., T.J.K. Radovich, N. V. Hue, S. T. Talcott, and K. A. Krenek. 2009. Vermicompost extracts influence growth, mineral nutrients, phytonutrients and antioxidant activity in pak choi (Brassica Rapa Cv. Bonsai, Chinensis Group) grown under vermicompost and chemical fertiliser. Journal of the Science of Food and Agriculture 89:2383—2392. (Available online at: https://doi.org/10.1002/jsfa.3732 (verified 14 Aug 2019).
- Parr, J. F., and R. I. Papendick. 1978. Factors affecting the decomposition of crop residues by microorganisms. Page 101–129. In W. R. Oschwald (ed.) Crop Residue Management Systems. American Society of Agronomy Special Publication 31. ASA, CSSA, and SSSA, Madison, WI.
- Puigde la Bellacasa, María. 2014. Encountering bioinfrastructure: Ecological struggles and the sciences of soil. Social Epistemology 28(1):26–40. (Available online at: https://doi.org/10.1080/02691728.2013.862879
- Rzewnicki, P. 1992. EC92-125 On-farm trials for farmers using the randomized complete block design [Online]. Historical Materials from University of Nebraska-Lincoln Extension. 1558. Available at: http://digitalcommons.unl.edu/extensionhist/1558 (verified 14 Aug 2019).
- Santos, P. F., J. Phillips, and W. G. Whitford. 1981. The role of mites and nematodes in early stages of buried litter decomposition in a desert. Ecology 62:664—669. (Available online at: https://doi.org/10.2307/1937734 (verified 14 Aug 2019).
- Scheuerell, S. J., and W. F. Mahaffee. 2005. Microbial recolonization of compost after peak heating needed for the rapid development of damping-off suppression. Compost Science & Utilization 13:65—71. (Available online at: https://doi.org/10.1080/1065657X.2005.10702219 (verified 14 Aug 2019).
- Wortmann, C. S., J. A. Quincke, R. A. Drijber, M. Mamo, and T. Franti. 2008. Soil microbial community change and recovery after one-time tillage of continuous no-till. Agronomy Journal 100:1681. (Available online at: https://doi.org/10.2134/agronj2007.0317 (verified 14 Aug 2019).
- Zinati, G. 2018. Chemically and biologically-designed compost extract: A potential tactic for biological control of weeds [Online]. Rodale Institute Research Articles. Available at: https://rodaleinstitute.org/science/articles/control-weeds-with-compost-extract/ (verified 14 Aug 2019).



