eOrganic author:
Dr. Mark Schonbeck, Virginia Association for Biological Farming
Abstract
Pigweed is the common name for several closely related summer annuals that have become major weeds of vegetable and row crops throughout the United States and much of the world. Most pigweeds are tall, erect-to-bushy plants with simple, oval- to diamond-shaped, alternate leaves, and dense inflorescences (flower clusters) comprised of many small, greenish flowers. They emerge, grow, flower, set seed, and die within the frost-free growing season.
Pigweeds thrive in hot weather, tolerate drought, respond to high levels of available nutrients, and are adapted to avoid shading through rapid stem elongation. They compete aggressively against warm season crops, and reproduce by prolific seed production.
In organic production systems, pigweeds can be managed through a combination of:
- Timely cultivation, flame weeding, and manual removal
- Stale seedbed
- Mulching
- Crop rotations that vary timing of tillage and other operations
- Cover crops and competitive cash crops
- Measures to prevent or minimize production of viable seeds
Introduction
Virtually every farmer in North America knows and grapples with pigweed, a term that covers several species in the genus Amaranthus, including:
- redroot pigweed (A. retroflexus)
- smooth pigweed (A. hybridus)
- Powell amaranth (A. powelii)
- Palmer amaranth (A. palmeri)
- spiny amaranth (A. spinosus)
- tumble pigweed (A. albus)
- prostrate pigweed (A. blitoides)
- waterhemp (A. tuberculatus = A. rudis)
These heat-loving summer annuals emerge after the spring frost date, grow rapidly, compete vigorously against warm-season crops, reproduce by seed, and die with the fall frost. Pigweeds are major weeds of warm season vegetables (Webster, 2006) and row crops (Sellers et al., 2003).
Also called amaranths, pigweeds are native to parts of North and Central America. Crop cultivation and human commerce have opened new niches, allowing pigweeds to invade agricultural ecosystems throughout the Americas, and parts of Europe, Asia, Africa, and Australia. Most amaranths make nutritious green vegetables or grain crops, and deliberate planting for food has helped some weedy species spread around the world. However, none of the pigweeds discussed here is grown commercially for grain, and modern grain amaranth varieties are not considered major agricultural weeds.
Pigweed problems have increased in no-till production systems with conventional herbicides, which leave weed seeds at the surface and select for herbicide-resistant populations (Sellers et al., 2003). However, high pigweed populations can occur on organic and non-organic farms, and in conventional, conservation, and no-till systems.
Description and Identification
Pigweeds are easy to recognize, yet correct identification of pigweed species can be tricky. Two or more pigweed species often occur together in the same field (Fig. 1), significant variation can occur within a species, and interspecific hybrids occasionally occur (Sellers et al., 2003). Some researchers consider tall waterhemp and common waterhemp a single species: A. tuberculatus (Pratt and Clark, 2001). Kansas State University Extension has published an excellent pigweed identification guide with photo illustrations and a key to distinguish mature plants of nine different weedy amaranths (Horak et al., 1994).

Figure 1. Two pigweed species, tentatively identified as Palmer amaranth (left) and smooth pigweed (right), grow at the edge of a plastic mulched bed in organic vegetable production in Clemson, South Carolina. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Newly emerging pigweed seedlings open a pair of long, narrow cotyledons, about 0.5 inch long by 0.1 inch wide, followed by the first true leaves, which are broader in outline (Fig. 2). Plants form moderately deep, branching taproots, and may show a distinct reddish coloration on roots, lower stems, and undersides of leaves.

Figure 2. In this flush of summer annual weed seedlings, pigweed (Amaranthus sp.) can be distinguished by its pair of long, narrow cotyledons (seed leaves), and, on older seedlings, true leaves that are much more broadly oval in outline. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Most pigweeds grow into large, erect-to-bushy plants, 2–7 feet in height, with simple, petiolate (stalked) leaves arranged alternately (singly) on stems (Fig. 3a). Leaf blades are generally oval-to-diamond shaped, and 2–6 inches long. Prostrate pigweed forms a low, spreading mat, with smaller (about one inch) leaves that are distinctly notched at the tip (Fig. 3b).

Figure 3. a. These smooth pigweeds in early heading are about four feet tall. b. Prostrate pigweed forms a low, spreading mat. Photo credits: Mark Schonbeck, Virginia Association for Biological Farming.
Individual pigweed flowers are small, inconspicuous, and usually greenish in color. Male and female flowers are borne on the same plant (most species) or separate plants (waterhemp, Palmer amaranth). Each plant bears thousands of flowers in small clusters in leaf axils, or larger, often branched, densely-packed spikes at the tips of main stems and major branches (Fig. 4). Female flowers form single, small, round, usually shiny, dark reddish-brown-to-black seeds, roughly 0.04 inch in diameter (Fig. 5). About 50,000–90,000 seeds weigh one ounce.

Figure 4. Spiny amaranth (left) and smooth pigweed (right) in bloom. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
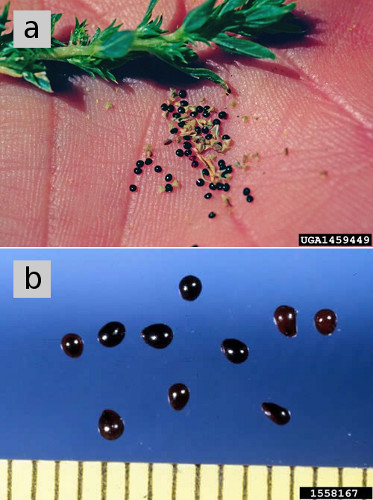
Figure 5. (a) Seeds of tumble pigweed. (b) Seeds of redroot pigweed, magnified, showing dark, shiny seed coat of mature seeds. Figure credits: (a) Steve Dewey, Utah State University, Bugwood.org. (b) Ken Chamberlain, Ohio State University, Bugwood.org.
See Table 1 below for a quick guide to eight common North American pigweed species, with links to additional information about each.
| Common and Scientific Name | Growth Habit | Inflorescence* | Geographic Range** | Other Plant Characteristics |
|---|---|---|---|---|
| Redroot Pigweed Amaranthus retroflexus | Erect, branched, 2–7 ft | Stiff, branched terminal spikes, individual branches usually <2 in long, thicker than pencil | Throughout North America including Alaska | Upper stem and leaves usually covered with fine hairs; leaf blades large (6 in) on vigorous plants |
| Smooth Pigweed Amaranthus hybridus | Erect, branched, 2–7 ft | Soft, highly branched terminal spikes, individual branches thinner than pencil | Throughout North America | Similar to redroot but highly variable, many local variants, may hybridize with closely related species |
| Palmer Amaranth Amaranthus palmeri | Erect, branched, 2–10 ft | Long (to 18 in), simple or sparingly branched terminal spikes; male soft, female bristly | Southern half of U.S., Great Plains, Mexico | Extremely rapid, aggressive growth in hot climates, male and female flowers on separate plants; plants smooth and hairless |
| Powell Amaranth Amaranthus powellii | Erect, branched, 2–6 ft | Stiff, branched terminal spikes, branches 4–8 in long, thicker than pencil, held close to main axis | Throughout North America | First true leaves narrower and more tapered toward tip than redroot or smooth; plant may be smooth or hairy |
| Spiny Amaranth Amaranthus spinosus | Erect to bushy 1–4 ft | Slender, branched terminal spikes mostly male flowers; axillary clusters mostly female | Throughout North America, but mostly Southeastern U.S. | Pair of stiff, sharp ½-in spines at base of each leaf; stems smooth, hairless, often red |
| Waterhemp Amaranthus rudis or A. tuberculatus*** | Erect, tall 3–10 ft | Slender, simple or branched terminal spikes | Throughout U.S. and southern Canada except driest areas | Male and female flowers on separate plants; stems and leaves smooth and hairless; leaves often longer and narrower than other species |
| Prostrate Pigweed Amaranthus blitoides | Prostrate mat to 3 ft across | Small, dense clusters in leaf axils | Throughout U.S. and southern Canada | Leaves small (blade about 1 in) with distinct notch at tip; seeds dull black, larger than in other pigweeds (0.06 in) |
| Tumble Pigweed Amaranthus albus | Globular bush, 1–3 ft diameter | Small, dense clusters in leaf axils | Throughout North America | Mature plants break off at ground level, and are carried by wind, dispersing seeds; stems white to pale green, leaves light green |
| * Small clusters of flowers are usually present in leaf axils of all amaranths ** Within North America (Canada, U.S., Mexico); many species have become naturalized on other continents. *** Some authors recognize two species, common waterhemp (A. rudis) and tall waterhemp (A. tuberculatus); others consider them subspecies, or synonymous. | ||||
Life Cycle, Reproduction, Seed Dispersal, Seed Dormancy, and Germination
Pigweeds are frost-tender summer annuals that emerge, grow, flower, and form mature seed within the frost-free period. Seedlings emerge over an extended period, with major flushes in late spring or early summer (Fig. 6). In most species, flowering and seed development take place mainly after the summer solstice, in response to shortening daylengths.
Figure 6. A flush of smooth pigweed seedlings on a vegetable farm in the Tidewater region of Virginia, photographed on June 20, 2010, about two weeks after emergence. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Pigweeds reproduce entirely by seed. A single large plant can mature 100,000–600,000 seeds, and populations of 0.1–1 plants per square foot can shed 10,000–45,000 seeds per square foot, or 0.4–2 billion per acre (Massinga et al., 2001; Sellers et al., 2003). This prolific seed production makes pigweeds especially difficult to manage, since successful maturation of just one plant per 10,000 emerging seedlings can allow pigweed populations to increase severalfold from one year to the next.
Pigweeds typically begin to flower and shed pollen (anthesis) about six weeks after emergence (WAE), although flowers can occur as early as 3 WAE or as late as 9 WAE (Huang et al., 2000; Keeley, et al., 1987; Shrestha and Swanton, 2007). Flowers open about 1–2 weeks after flower buds first become visible to the unaided eye.
Reported time intervals from pollination to formation of viable seeds range from 7–12 days in waterhemp (Bell and Tranel , 2010) to 6 weeks in field populations of redroot pigweed in Ontario (Shrestha and Swanton, 2007). In California, Palmer amaranth formed viable seeds 2–6 weeks after flowering (Keeley et al., 1987). Seeds become viable at about the same time that they develop their mature dark brown or black color.
Reproductive development is accelerated by shortening daylength after the summer solstice in field populations (Keeley et al., 1987), and proceeds faster in short (~12 hour) than in longer (≥14 hour) photoperiods in a growth chamber (Huang et al., 2000). Although most seed production occurs in late summer and early fall, some mature seeds have been found in smooth pigweed seed heads at the summer solstice in Virginia (personal observation).
The ability of pigweed plants uprooted or severed at flowering to complete seed maturation has not been researched. However, in upstate New York, 2–4-inch fragments of Powell amaranth inflorescences lying on the soil surface were found to contain black seeds 3 weeks after the weeds were disked down at flowering (Charles Mohler, Cornell University, pers. commun.). Apparently, if pollination takes place before pigweeds are pulled or chopped, some potential exists for viable seed production.
Pigweed seeds are dispersed to new locations by irrigation or flood water, manure, and soil clinging to footwear, tractor tires, or tillage tools. In addition, tumble pigweed actively disperses seeds when mature plants break off and move with the wind.
Pigweed seeds have multiple dormancy mechanisms, so that seeds produced in a given season germinate at different times over the next several years, thereby enhancing the weed's long-term persistence (Egley, 1986). Newly shed pigweed seeds are mostly dormant, and become less so by the following spring. Germination is promoted by high temperatures (95 °F), diurnally fluctuating temperatures (e.g., 85–95 °F day, ~ 70 °F night), and sometimes light (Guo and Al-Khatib, 2003; Schonbeck and Egley, 1980 and 1981 Steckel et al., 2004).
Pigweed emerges most readily from the top 0.5–1.0 inch of the soil profile, with few emerging from seeds located deeper than one inch (Mohler and Di Tommaso, unpublished). The seeds require adequate moisture and good seed–soil contact to absorb moisture and germinate. More deeply buried seeds remain dormant and viable for several years, and germinate when brought to the surface by tillage or cultivation. Although flushes of emergence commonly follow seedbed preparation or cultivation, increasing pigweed problems in agronomic crops have been attributed to widespread adoption of no-till and minimum-tillage, which leave recently-shed weed seeds at or near the soil surface (Sellers et al., 2003).
Growth Habit and Impact on Crops
Pigweeds have the C4 photosynthetic pathway, which confers an ability to grow rapidly at high temperatures and high light levels, to tolerate drought, and to compete aggressively with warm-season vegetables for light, moisture, and nutrients. Growth is related to cumulative Growing Degree Days, with a base temperature of 50 °F (Shrestha and Swanton, 2007; Horak and Loughin, 2000); thus, pigweeds grow much faster in hot climates than in northern regions with cooler summers.
Erect pigweed species can rapidly overtop short crops like broccoli or snap bean. In taller crops like corn, pigweeds respond to canopy shade by increasing stem growth and deploying leaves higher on the plant, thereby intercepting a larger fraction of available light (Massinga et al., 2003; McLachlan et al, 1993). One to three pigweed plants per 10 feet of row emerging with corn or soybean can cause significant yield losses (Klingman and Oliver, 1994; Knezevic et al., 1994; Massinga et al., 2001) Pigweeds that emerge several weeks after the crop has emerged exert much less effect on yields.
Pigweeds are highly responsive to nutrients, especially the nitrate form of nitrogen (N) (Blackshaw and Brandt, 2008; Teyker et al., 1991). Fertilization enhances both weed biomass and seed production. In addition, nitrate can stimulate pigweed seed germination (Egley, 1986). A mulch of legume cover crop residues has been observed to enhance pigweed emergence in some years (Fig. 7), likely as a result of rapid mineralization of legume N (Teasdale and Mohler, 2000).

Figure 7. In this field trial, a flush of pigweed competes against broccoli planted no-till into killed hairy vetch (foreground), while broccoli planted in killed rye or rye–vetch are relatively free of pigweed (background). Rapid N mineralization from the all-legume cover crop residues apparently stimulated pigweed germination and growth. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Because the small seeds have minimal nutrient reserves, pigweed seedlings are initially more dependent on readily available nutrients from the soil, especially phosphorus (P) and potassium (K), than larger-seeded plants such as corn, beans, and cucurbits (Hoveland et al., 1976; Mohler, 1996). However, in studies conducted on organic (muck) soils in Florida, smooth pigweed and spiny amaranth were less responsive than lettuce to P levels, and a band application of P fertilizer improved the crop's ability to compete against these weeds (Santos et al., 1997; Shrefler et al., 1994).
Pigweeds are shade intolerant, and the growth and reproduction of individuals that emerge under a heavy crop canopy are substantially reduced. However, rapid stem elongation allows pigweeds to escape shading in many cropping situations. Late-season pigweeds that break through established cucurbit, tomato, pepper, and other vegetables can promote crop disease by reducing air circulation, interfere with harvest, and set many thousands of seeds (Fig. 8).

Figure 8. The pigweed emerged several weeks after squash planting and did not affect yield. By the end of crop harvest, however, each weed matured thousands of seeds, and will make a heavy deposit into the weed seed bank unless they are removed promptly. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Pigweeds are reported to host pest nematodes (Meloidogyne spp.) and many vegetable crop pathogens, including fungi that cause early blight in potato and tomato (Alternaria solani), lettuce drop (Sclerotinia sclerotiorum) and southern blight (Sclerotium rolfsii) in a wide range of crops. Viral pathogens such as cucumber mosaic virus and tomato spotted wilt virus can also be transmitted from pigweeds (Mohler and DiTommaso, unpublished).
Pigweeds have become the focus of biocontrol efforts with fungal pathogens and plant-feeding insects, although no biocontrol products have yet become available to farmers. The amaranth flea beetle (Disonycha glabrata) occurs throughout much of the United States (Tisler, 1990), feeds on pigweed foliage (Fig. 9), and may become a significant natural enemy of pigweed in some areas, including Floyd County, Virginia (personal observation). However, it usually does not control the weeds, and occasionally feeds on some vegetable seedlings.

Figure 9. The amaranth flea beetle feeds on pigweed foliage, and has been observed to cause substantial defoliation and reduce weed vigor in some parts of Virginia. This insect can occasionally become a pest in beet and chard by feeding on seedlings. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Management
Organic farmers manage pigweeds by taking advantage of their points of vulnerability. The small seeds have minimal nutrient reserves; thus seedlings can emerge only from seeds located within an inch of the soil surface, and are immediately dependent on the soil for readily available nutrients. Transplanted and large-seeded crops have substantial nutrient reserves, and can gain a competitive edge over pigweed seedlings if slow-release nutrient sources are used.
The delicate seedlings are readily killed by severing, uprooting, burial, or heat. Timely flame weeding or cultivation with any of a variety of implements can knock out a flush of pigweed seedlings. Emerging pigweed is also susceptible to shading and physical hindrance by mulch. A field study at Beltsville, Maryland documents the greater sensitivity of pigweed to suppression with organic mulches relative to several other common weeds: redroot pigweed > lamb's quarter > giant foxtail > velvetleaf (Teasdale and Mohler, 2000).
Timely action is vital, as pigweeds rapidly become harder to kill once they grow taller than one inch and develop four or more true leaves (Fig. 10). In cool climates, pigweed seedlings may remain vulnerable to cultivation for up to 4 WAE (Weaver and McWilliams, 1980); however in warmer climates, they can grow to 2–4 inches within 2 WAE (Sellers et al., 2003).

Figure 10. The pigweed seedling on the right is at the vulnerable stage, at which it can be readily killed by shallow cultivation or flaming, or blocked by mulch. When pigweed grows as large as the seedling on the left, it becomes more difficult to kill, requiring more vigorous cultivation. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Pigweed populations readily adapt to production systems and control tactics. For example, seed germination responses show adaptive changes to different crop rotations (Brainard et al., 2007), and widespread herbicide resistance has been reported in several species (Fugate, 2009; Volenberg et al., 2007). Thus, reliance on a single management tool or the same strategy year after year will likely yield diminishing returns over time.
When used in combination, the practices described below can provide effective management of pigweed in organic systems.
Cultivation and Flame Weeding
Monitor crops regularly for weed emergence. Cultivate when pigweeds are in the cotyledon stage, or before they reach one inch in height, working as close to the crop row as practical. When the crop is sufficiently established, set cultivators to move an inch or so of soil into rows to bury small weeds. The surface layer of loose, dry soil left by cultivation (dust mulch) deters additional pigweed germination. Avoid recompacting the soil, as compaction can promote another flush of emergence (Fig. 11).

Figure 11 Cultivation left a dust mulch around these young squash plants, thereby discouraging germination of pigweed and other small-seeded weeds. However, foot traffic recompacted the soil enough to re-establish seed–soil contact near the surface, thereby allowing weed seeds to imbibe moisture, germinate, and grow in the footprints. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Flame weeding can remove pigweed and other broadleaf seedlings just before crop emergence. It is often used for slow-starting crops like carrot, beet, and parsnip. Because flaming usually does not kill grass weed seedlings, it is not recommended where grasses make up a significant portion of the weed flora.
Mowing and Grazing
Once the crop is too large to cultivate by tractor, farmers often mow, cut, or pull weeds in alleys to maintain air circulation around the crop, facilitate harvest, and prevent weed propagation. This should be done before pigweed flowers open (within a few days after flower heads first become visible) to prevent viable seed formation.
Some farmers mow alleys between wide rows or plastic mulched beds with a push mower or line trimmer as a soil-saving alternative to cultivation. Two timely mowings prior to canopy closure have given adequate between-row control of giant foxtail, pigweeds, and ragweed in soybean planted in a 30-inch row spacing (Donald, 2000).
Most pigweeds are highly palatable to livestock. However, mature seeds pass through the animals' digestive tracts unharmed, and manure is a notorious source of pigweed seeds. Thus, pigweed should be grazed while still vegetative. Note also that the National Organic Program requires a 120-day interval between manure deposits by grazing animals and the next food-crop harvest.
Mulching
Mulching can be an effective control tactic for pigweeds in vegetable production. An organic mulch, such as 3–4 inches of straw or hay (~5–10 tons/ac), applied within a day after cultivating an established crop, can reduce subsequent pigweed emergence by 90%. Alternatively, a synthetic mulch such as black plastic can be laid before crop planting, and alley weeds controlled by cultivation, mowing, organic mulch, or cover crop. Note: If plastic or other synthetic mulch is used to organic crops, it must be removed from the field at the end of the harvest or growing season.
Organic no-till transplanting of tomato and other summer vegetables into roll–crimped or mowed winter cover crops can control light-to-moderate pigweed populations. Rye residues release natural plant growth inhibitors (allelochemicals) that suppress pigweed and some other annual weeds (Barnes and Putnam, 1983; Putnam et al., 1983) without affecting transplanted vegetables.
Nutrient and Moisture Management
Use slow-release sources of N and other crop nutrients, and avoid broadcast application of faster-release materials like blood meal and bone meal, which can give pigweed the jump on the crop. For heavy feeders like broccoli or spinach that need some quick N, band or side dress materials within or near the crop row at the onset of rapid crop growth.
Use in-row drip irrigation to provide water and liquid organic fertilizer directly to the crop without feeding and watering between-row weeds. Subsurface drip lines can provide moisture to the crop and leave the soil surface dry, thereby minimizing within-row weed emergence.
Crop Rotation, Planting Schedules, and Stale Seedbed
Plan crop rotation and schedule field operations to disrupt pigweed life cycles. Avoid providing an open niche (bare soil) year after year for pigweed emergence in late spring to early summer. Alternate warm- and cool-season vegetables. Consider delaying seedbed preparation for a summer vegetable until after the time of peak pigweed emergence. After several years of intensive vegetable production, rotate the field to perennial sod (e.g., orchardgrass–red clover) for two or three years to disrupt pigweed life cycles and encourage weed seed predation.
If pigweed populations are high (Fig. 12), prepare a stale seedbed in late spring to draw down the weed seed bank. Till or cultivate, then roll or cultipack the soil to improve seed–soil contact, thereby promoting weed germination. Sprinkle irrigate if the soil is dry. Repeat cultivation as needed. Just before crop planting or crop emergence, use shallow cultivation and leave the surface loose to discourage additional weed germination. The final flush can also be killed by flame if grass weeds are few or absent.

Figure 12. A carpet of spiny amaranth seedlings arises from a large weed seed bank. A stale seedbed or cultivated fallow is needed to bring this situation under control. Photo credit: Mark Schonbeck, Virginia Association for Biological Farming.
Crop Competition and Cover Cropping
With good early-season weed control, vigorous crops like tomato, sweet potato, and winter squash can tolerate later-emerging pigweed. However, crop competition may not control pigweeds, because of their shade-avoidance response and ability to break through the crop canopy through rapid stem elongation.
Competitive summer cover crops such as buckwheat, sorghum–sudangrass, cowpea, and forage soybean are often used to suppress weeds between spring and fall vegetable crops. In Florida, cowpea, sunnhemp, or velvetbean cover crops seeded at high rates reduced but did not eliminate smooth pigweed growth (Collins et al., 2008).
When using summer cover crops to combat pigweeds, seed at high rates (1.5–2 times normal), and use good seeding methods to obtain a weed-suppressive cover crop stand. Combine cowpea, forage soybean, or other summer legume with a tall grass like pearl millet or sorghum–sudangrass to develop a canopy that is both tall and dense. Watch the crop closely; if a significant amount of pigweed grows with it, terminate the crop promptly when weed flower heads first appear.
Managing the Pigweed Seed Bank
Because pigweeds produce seed so prolifically, it is critical to minimize the annual seed rain onto the soil. Although stringent weed control for six years can reduce the pigweed seed bank by 99%, relaxing weed control allows seed numbers to recover to near original levels within three years (Schweizer and Zimdahl, 1984). Pigweed that emerges after a crop's minimum weed-free period may not reduce the crop's yield, but it should be pulled or cut before flowering to prevent formation of mature seeds.
It may pay to walk fields of maturing crops to pull or chop out large weeds; small, stunted pigweeds below a crop canopy form only small numbers of seeds. If flower heads are already formed, remove severed or uprooted pigweed plants from the field. If pigweed plants have already formed seed, note that many of the seeds will stay in the head until winter. Therefore, removing the weeds in early fall can still significantly reduce the pigweed seed rain.
In the event that a heavy pigweed seed rain occurs, some weed scientists recommend inversion tillage to move seeds to a depth from which they cannot emerge (Mohler and Di Tommaso, unpublished). Although 5–14% of redroot pigweed and waterhemp seeds have survived 9–12 years burial at 8-inch depth in Nebraska (Burnside et al., 1996), others have reported that pigweed seeds are fairly short lived (3–4 years) in the soil in more humid regions such as Mississippi and Illinois (Buhler and Hartzler, 2001; Egley and Williams, 1990; Steckel et al., 2007). Moldboard plowing has been reported to increase pigweed emergence if weed populations are low, but to decrease emergence if populations are high as a result of a recent seed rain (Schweizer and Zimdahl, 1984).
When using inversion tillage to manage a heavy seed deposit, moldboard plow the field once, then avoid deep tillage for the next several years to allow buried seeds to lose viability.
This article is part of a series discussing the invasive family of Pigweeds. For more information, see the following articles:
- Weed Profile: Pigweeds (Amaranthus spp.)
- Redroot Pigweed (Amaranthus retroflexus)
- Powell Amaranth (Amaranthus powellii)
- Spiny Amaranth (Amaranthus spinosus)
- Palmer Amaranth (Amaranthus palmeri)
- Smooth Pigweed (Amaranthus hybridus)
- Tumble Pigweed (Amaranthus albus)
- Prostrate pigweed (Amaranthus blitoides)
- Common Waterhemp (Amaranthus rudis) and Tall Waterhemp (A. tuberculatus)
References Cited
- Barnes, J. P., and A. R. Putnam. 1983. Rye residues contribute weed suppression in no-tillage cropping systems. Journal of Chemical Ecology 9: 1045–1057. (Available online at: http://dx.doi.org/10.1007/BF00982210) (verified 10 Sept 2012).
- Bell, M. S., and P. J. Tranel. Time requirement from pollination to seed maturity in waterhemp (Amaranthus tuberculatus). Weed Science 58: 167–173. (Available online at: http://dx.doi.org/10.1614/WS-D-09-00049.1) (verified 10 Sept 2012).
- Blackshaw, R. E., and R. N. Brandt. 2008. Nitrogen fertilizer rate effects on weed competitiveness is species dependent. Weed Science 56: 743–747. (Available online at: http://dx.doi.org/10.1614/WS-08-065.1) (verified 10 Sept 2012).
- Brainard, D. C., A. DiTommaso, and C. A. Mohler. 2007. Intraspecific variation in seed characteristics of Powell amaranth (Amaranthus powellii) from habitats with contrasting crop rotation histories. Weed Science 55: 218–226. (Available online at: http://dx.doi.org/10.1614/WS-06-134.1) (verified 10 Sept 2012).
- Buhler, D. D. and R. G. Hartzler. 2001. Emergence and persistence of seed of velvetleaf, common waterhemp, woolly cupgrass, and giant foxtail. Weed Science 49: 230–235. (Available online at: http://dx.doi.org/10.1614/0043-1745(2001)049%5B0230:EAPOSO%5D2.0.CO;2) (verified 10 Sept 2012).
- Burnside, O. C., R. G. Wilson, S. Weisberg, and K. G. Hubbard. 1996. Seed longevity of 41 weed species buried 17 years in eastern and western Nebraska. Weed Science 44: 74–86. (Available online at: http://www.jstor.org/stable/4045786) (verified 10 Sept 2012).
- Collins, A. S., C. A. Chase, W. M. Stall, and C. M. Hutchinson. 2008. Optimum densities of three leguminous cover crops for suppression of smooth pigweed (Amaranthus hybridus). Weed Science 56: 753–761. (Available online at: http://dx.doi.org/10.1614/WS-07-101.1) (verified 10 Sept 2012).
- Donald, W. W. 2000. Between-row mowing + in-row band-applied herbicide for weed control in Glycine max. Weed Science 48: 487–500. (Available online at: http://www.jstor.org/stable/4046280) (verified 10 Sept 2012).
- Egley, G. H. 1986. Stimulation of weed seed germination in soil. Reviews of Weed Science 2: 67–89.
- Egley, G. H., and R. D. Williams. 1990. Decline of weed seeds and seedling emergence over five years as affected by soil disturbances. Weed Science 38: 504–510. (Available online at: http://www.jstor.org/stable/4045064) (verified 10 Sept 2012).
- Fugate, L. 2009. Pigweed causing farmers to rethink farming methods. University of Arkansas Division of Agriculture Cooperative Extension Service News - October 2009.
- Guo, P., and K. Al-Khatib. 2003. Temperature effects on germination and growth of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis). Weed Science 51: 869–875. (Available online at: http://dx.doi.org/10.1614/P2002-127) (verified 10 Sept 2012).
- Horak, M. J., and T. M. Loughin. 2000. Growth analysis of four Amaranthus species. Weed Science 48: 347–355. (Available online at: http://dx.doi.org/10.1614/0043-1745(2000)048%5B0347:GAOFAS%5D2.0.CO;2) (verified 10 Sept 2012).
- Horak, M. J., D. E. Peterson, D. J. Chessman, and L. M. Wax. 1994. Pigweed identification: a Pictoral guide to the common pigweeds of the Great Plains. 12 pp. (Available online at: http://www.ksre.ksu.edu/bookstore/pubs/S80.pdf) (verified 6 Aug 2013).
- Hoveland, C. S., G. A. Buchanan, and M. C. Harris. 1976. Response of weeds to soil phosphorus and potassium. Weed Science 24: 194–201. (Available online at: http://www.jstor.org/stable/4042586) (verified 10 Sept 2012).
- Huang, J. Z., A. Shrestha, M. Tollenar, W. Deen, H. Rahimian, and C. J. Swanton. 2000. Effect of photoperiod on the phenological development of redroot pigweed (Amaranthus retroflexus L.). Canadian Journal of Plant Science 80: 929–938.
- Keeley, P. E., C. H. Carter, and R. J. Thullen. 1987. Influence of planting date on growth of Palmer amaranth (Amaranthus palmeri). Weed Science 35: 199–204. (Available online at: http://www.jstor.org/stable/4044391) (verified 10 Sept 2012).
- Klingman, T. E., and L. R. Oliver. 1994. Palmer amaranth (Amaranthus palmeri) interference in soybeans (Glycine max). Weed Science 42: 523–527. (Available online at: http://www.jstor.org/stable/4045448) (verified 10 Sept 2012).
- Knezevic, S. Z., S. F. Weise, and C. J. Swanton. 1994. Interference of redroot pigweed (Amaranthus retroflexus) in corn (Zea mays). Weed Science 42: 568–573. (Available online at: http://www.jstor.org/stable/4045456) (verified 10 Sept 2012).
- Massinga, R. A., R. S. Currie, M. J. Horak, and J. Boyer, Jr. 2001. Interference of Palmer amaranth in corn. Weed Science 49: 202–208. (Available online at: http://dx.doi.org/10.1614/0043-1745(2001)049%5B0202:IOPAIC%5D2.0.CO;2) (verified 10 Sept 2012).
- Massinga, R. A., R. S. Currie, and T. P. Trooien. 2003. Water use and light interception under Palmer amaranth (Amaranthus palmeri) and corn competition. Weed Science 51: 523–531. (Available online at: http://dx.doi.org/10.1614/0043-1745(2003)051%5B0523:WUALIU%5D2.0.CO;2) (verified 10 Sept 2012).
- McLachlan, S. M., M. Tollenaar, C. J. Swanton, and S. F. Weise. 1993. Effect of corn-induced shading on dry matter accumulation, distribution, and architecture of redroot pigweed (Amaranthus retroflexus). Weed Science 41: 568–573. (Available online at: http://www.jstor.org/stable/4045424) (verified 10 Sept 2012).
- Mohler, C. A. 1996. Ecological bases for the cultural control of annual weeds. Journal of Production Agriculture 9: 468–474..
- Mohler, C. A., and A. DiTommaso. Unpublished. Manage Weeds on Your Farm: a Guide to Ecological Strategies. Department of Crop and Soil Sciences, Cornell University. Pre-publication draft, version 5.1. Publication anticipated in 2012.
- Pratt, D. B., and L. G. Clark. 2001. Amaranthus rudis and A. tuberculatus—one species or two? Journal of the Torrey Botanical Society 128: 282–296. (Available online at: http://www.jstor.org/stable/3088718) (verified 10 Sept 2012).
- Putnam, A. R., J. DeFrank, and J. P. Barnes. 1983. Exploitation of allelopathy for weed control in annual and perennial cropping systems. Journal of Chemical Ecology 9: 1001–1010. (Available online at: http://dx.doi.org/10.1007/BF00982207) (verified 10 Sept 2012).
- Santos, B. M., J. A. Dusky, D. G. shilling, W. M. Stall, and T. A. Bewick. 1997. Effect of phosphorus fertility on competitive interactions of smooth pigweed (Amaranthus hubridus), spiny amaranth (Amaranthus spinosus), and common purslane (Portulaca oleracea) with lettuce. Weed Science Society of America Abstracts 37: 54.
- Schonbeck, M. W., and G. H. Egley. 1980 Redroot pigweed (Amaranthus retroflexus) seed germination responses to afterripening, temperature, ethylene, and some other environmental factors. Weed Science 28: 543–548. (Available online at: http://www.jstor.org/stable/4043277) (verified 10 Sept 2012).
- Schonbeck, M. W., and G. H. Egley. 1981. Changes in sensitivity of Amaranthus retroflexus L. seeds to ethylene during preincubation. II. Effects of alternating temperature and burial in soil. Plant, Cell, and Environment 4: 237–242. (Available online at: http://dx.doi.org/10.1111/1365-3040.ep11611005) (verified 10 Sept 2012).
- Schweizer, E. E., and R. L. Zimdahl. 1984. Weed seed decline in irrigated soil after six years of continuous corn (Zea mays) and herbicides. Weed Science 32: 76–83. (Available online at: http://www.jstor.org/stable/4043886) (verified 10 Sept 2012).
- Sellers, B. A., R. J. Smeda, W. G. Johnson, J. A. Kendig, and M. R. Ellersieck. 2003. Comparative growth of six Amaranthus species in Missouri. Weed Science 51: 329–333. (Available online at: http://dx.doi.org/10.1614/0043-1745(2003)051%5B0329:CGOSAS%5D2.0.CO;2) (verified 10 Sept 2012).
- Shrefler, J. W., J. A. Dusky, D. G. Shilling, B. J. Brecke, and C. A. Sanchez. 1994. Effect of phosphorus fertility on competition between lettuce (Lactuca sativa) and spiny amaranth (Amaranthus spinosus). Weed Science 42: 556–560. (Available online at: http://www.jstor.org/stable/4045454) (verified 10 Sept 2012).
- Shrestha, A., and C. J. Swanton. 2007. Parameterization of the phenological development of select annual weeds under noncropped field conditions. Weed Science 55: 446–454. (Available online at: http://dx.doi.org/10.1614/WS-06-176.1) (verified 10 Sept 2012).
- Steckel, L. E., C. L. Sprague, E. W. Stoller, and L. M. Wax. 2004. Temperature effects on germination of nine Amaranthus species. Weed Science 52: 217–221. (Available online at: http://dx.doi.org/10.1614/WS-03-012R) (verified 10 Sept 2012).
- Steckel, L. E., C. L. Sprague, E. W. Stoller, L. M. Wax, and F. W. Simmons. 2007. Tillage, cropping system, and soil depth effects on common waterhemp (Amaranthus rudis) seed-bank persistence. Weed Science 55: 235–239. (Available online at: http://dx.doi.org/10.1614/WS-06-198) (verified 10 Sept 2012).
- Teasdale, J. R., and C. L. Mohler. 2000. The quantitative relationship between weed emergence and the physical properties of mulches. Weed Science 48: 385–392. (Available online at: http://dx.doi.org/10.1614/0043-1745(2000)048%5B0385:TQRBWE%5D2.0.CO;2) (verified 10 Sept 2012).
- Teyker, R. H., H. D. Hoelzer, and R. A. Liebl. 1991. Maize and pigweed response to nitrogen supply and form. Plant and Soil 135: 287–292. (Available online at: http://dx.doi.org/10.1007/BF00010918) (verified 10 Sept 2012).
- Tisler, A. M. 1990. Feeding in the pigweed flea beetle, Disonycha glabrata Fab. (Coleoptera: Chrysomelidae), on Amaranthus retroflexus. Virginia Journal of Science 41: 243–245.
- Volenberg, D. S., W. L. Patzoldt, A. G. Hager, and P. J. Tranel. 2007. Responses of contemporary and historical waterhemp (Amaranthus tuberculatus) accessions to glyphosate. Weed Science 55: 327–333. (Available online at: http://dx.doi.org/10.1614/WS-06-121) (verified 10 Sept 2012).
- Weaver, S. E., and E. L. McWilliams. 1980. The biology of Canadian weeds. 44. Amaranthus retroflexus L, A. powellii S. Wats and A. hybridus L. Canadian Journal of Plant Science 60: 1215–1234.
- Webster, T. M. 2006. Weed survey – southern states. Vegetable, fruit and nut crops subsection. Proceedings of the Southern Weed Science Society 59: 260–277. (Available online at: http://www.swss.ws/wp-content/uploads/docs/Southern%20Weed%20Survey%202006%20Vegetables%20and%20Fruits.pdf) (verified 10 Sept 2012).



